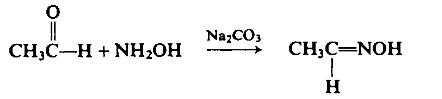| | Acetaldoxime Chemical Properties |
| Melting point | 44-46 °C | | Boiling point | 115 °C(lit.) | | density | 0.98 g/mL at 25 °C | | vapor pressure | 13 hPa (25 °C) | | refractive index | n20/D 1.426(lit.) | | Fp | 135 °F | | storage temp. | 2-8°C | | solubility | 185g/l | | form | Low Melting Solid or Liquid | | pka | 11.82±0.10(Predicted) | | color | White or clear | | explosive limit | 4.2-50%(V) | | Water Solubility | soluble | | Merck | 14,42 | | BRN | 1209252 | | LogP | -0.13 | | CAS DataBase Reference | 107-29-9(CAS DataBase Reference) | | NIST Chemistry Reference | Acetaldoxime(107-29-9) | | EPA Substance Registry System | Acetaldehyde, oxime (107-29-9) |
| | Acetaldoxime Usage And Synthesis |
| Overview | Acetaldehyde oxime is a reducing agent with low toxicity. In the 1990s, it replaced the highly toxic hydrazine as a boiler water deoxidizer. As its deoxidizing effect was forty times that of hydrazine, it became widely used as a new type of deoxidizer. Acetaldehyde oxime is also an important intermediate for synthesizing the pesticides Methomyl and Thiodicarb, two chemicals that are excellent large-scale pesticides. As the production of these pesticides continues to increase, so does the demand for acetaldehyde oxime.
| | Chemical properties | Colorless, needle-shaped crystals. There are two types of crystal structures: α-type and β-type. Relative molecular mass is 59.07. Relative density is 0.9656. Melting point is 46.5℃(α-type) and 12℃(β-type). Boiling point is 114.5℃. Refractive index is 1.4257. Very soluble in water, ethanol and ether. When heated with dilute acid, it will break down into acetaldehyde and hydroxylamine. Created by adding hydroxylamine to acetaldehyde aqueous solution. Used to identify or refine acetaldehyde. Avoid contact with strong oxidants and strong acids. Flammable and irritative to the eyes, respiratory system, and skin. Appropriate protective clothing should be worn when handled in large amounts. If it contacts the eyes, immediately rinse generously with water and seek medical attention.
| | Uses |
- Pesticide intermediate, synthesis of pesticides “Methomyl”, “Thiodicarb”, etc.; organic synthesis intermediate.
- When melted, excellent solvent for many inorganic and organic compounds, organic synthesis, stabilizer, plasticizer, alcohol denaturant.
- Creation of acylamide through the aldehyde oxime one-pot method. With the presence of catalyst InCl3, acetaldehyde oxime replaces water and reacts with nitrile water to create acylamide at a high yield.
| | Preparation | With suitable precautions, to a solution of 325 gm (4.68 moles) of hydroxylamine hydrochloride in 300 ml of water mixed with a solution of 255 gm (2.55 moles) of sodium carbonate in 600 ml of water, cooled in an ice-salt mixture, is added dropwise, with stirring, a solution of 200 gm (4.55 moles) of acetaldehyde in 100 ml of water. After standing overnight, the solution is saturated with sodium chloride and the product is separated by repeated extractions with ether.
The combined ether extracts are dried over calcium chloride, filtered, and distilled to afford 215 gm (80%), b.p. 112-114°C (760 mm Hg).
Because many aldehydes are not very water soluble, this reaction may be carried out either with vigorous stirring or by adding sufficient alcohol to make the reaction mixture homogeneous. When the product has to be purified by distillation, it has been recommended that the flask be immersed into an oil bath maintained at a constant temperature above the anticipated boiling point of the product rather than warming the product and oil bath up to the boiling range from room temperature. By this technique heptaldoxime, b.p. 103-107°C (66 mm Hg), m.p. 53-55°C, cyclohexanone oxime, b.p. 100-105°C (10-12 mm Hg), m.p. 87-88°C; and methyl ethyl ketoxime, b.p. 150-155°C, have been prepared. The treatment of a carbonyl compound with a hydroxylamine salt, with or without a solvent (or water), and with a neutralization step of the hydroxylamine salt using a simple base may be considered as a general preparative procedure.
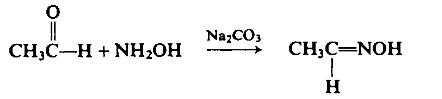
| | Storage |
- Keep in cool, ventilated storerooms, away from fire and heat sources. Storage heat should not exceed 30℃.Store separately from oxidants, acids and edible chemical substances – do not mix together. Employ explosion-proof lighting and ventilation facilities. Do not handle with machinery and tools that easily produce sparks. The storage area should have emergency measures for leaks and adequate storage equipment.
- Store in a sealed and dark container.
| | Preparation | Acetaldehyde oxime is produced through the reaction between hydroxylamine and acetaldehyde aqueous solution. The reaction equation is as follows: CH3CHO+NH2OH•HCl+NaOH→CH3CH=NOH+NaCl+H2O
In current industrial production, high levels of crystalline hydroxylamine sulfate are widely used as raw material, made into a 20%~50% aqueous solution and combined with acetaldehyde to create acetaldehyde oxime. The reaction equation is as follows: CH3CHO+(NH2OH)2•H2SO4→CH3CH=NOH
In the aforementioned addition of acetaldehyde into hydroxylamine sulfate aqueous solution, the reaction occurs for 2 hours in 40~50℃ temperature. After it is cooled, inorganic salts are removed to achieve a clear aqueous solution of approximately 50% acetaldehyde oxime. Through inspection, it is revealed that there are very few impurities in addition to acetaldehyde and water in the solution, and the yield of acetaldehyde oxime is above 90% (as hydroxylamine sulfate). Extraction and refinement of this solution can yield a product of over 90% acetaldehyde oxime.
| | Category | Flammable Liquid
| | Toxicity level | Highly toxic
| | Acute Toxicity | Abdominal injection – small mice LD50: 100Mg/kg
| | Explosivity characteristics | May explode when mixed with water vapor and air.
| | Flammability characteristics | Flammable; releases toxic nitrogen oxide gas at high temperatures
| | Storage and Transport | Ventilated, low-temperature and dry storage; store and ship separately from oxidants.
| | Extinguishing agents | Dry powder, carbon dioxide, sand, foam
| | Chemical Properties | WHITE LOW MELTING SOLID OR CLEAR LIQUID | | Chemical Properties | Acetaldehyde oxime is an extremely flammable, colorless liquid or crystalline solid; low melting crystalline compound. Pungent odor.
| | Uses | Acetaldoxime is used as an oxygen scavenger in boiler water. It is also used as an intermediate in chemical synthesis and pharmaceuticals. It is involved in the rearrangement reaction to prepare acetamide by using nickel(II) acetate as a catalyst. It acts as a precursor to prepare heterocyclic compound such as spiroisoxazoline. Further, it is used to prepare alkylated(Z)-oximes by deprotonation followed by reaction with benzyl bromide. | | Definition | The –CH:NOH radical resulting from reactions between aldehydes and hydroxylamine or by the oxidation of primary amines by persulfuric acid. | | General Description | A colorless liquid with a pungent odor. Density 0.966 g / cm3. Flash point 75°F. Boiling point 235°F. Has two crystalline modifications, one melting at 12°C and the other at 46.5°C. | | Air & Water Reactions | Highly flammable. Easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Very soluble in water. | | Reactivity Profile | Acetaldoxime may explode or decompose violently during distillation if samples have been previously been exposed to the air, which causes formation of peroxides of various types. Reacts as both a weak acid and as a weak base. Gives acetaldehyde and a hydroxylammonium salt if heated with aqueous acid. A nickel-catalyzed aldoxime rearrangement to an amide went out of control when a different solvent was employed [J. Loss Prev., 1993, 6(2), 69]. | | Health Hazard | May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. | | Fire Hazard | HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. | | Safety Profile | Poison via intraperitoneal route. Mutation data reported. A dangerous fire hazard with a flash point at room temperature. When heated to decomposition it emits toxic fumes of NOx. See also ALDEHYDES. | | Potential Exposure | Used as a chemical intermediate and as an antioxidant and radical scavenger with applications in many industries, including detergents, pharmaceuticals, plastics, paints and lacquers, rubber, and textiles | | Shipping | UN2332 Acetaldehyde oxime, Hazard Class: 3; Labels: 3-Flammable liquid. | | Incompatibilities | Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong acids (such as hydrochloric, sulfuric, and nitric), strong bases. The beta-form is able to form unstable peroxides | | Waste Disposal | Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed |
| | Acetaldoxime Preparation Products And Raw materials |
|