|
| | Trimethylolpropane Basic information |
| | Trimethylolpropane Chemical Properties |
| Melting point | 56-58 °C(lit.) | | Boiling point | 159-161 °C2 mm Hg(lit.) | | density | 1.176 | | vapor density | 4.8 (vs air) | | vapor pressure | <1 mm Hg ( 20 °C) | | refractive index | 1.4850 (estimate) | | Fp | 172 °C | | storage temp. | Store below +30°C. | | solubility | H2O: 0.1 g/mL, clear | | pka | 14.01±0.10(Predicted) | | form | Flakes | | color | White | | PH | 6.5 (100g/l, H2O, 20℃)(External MSDS) | | explosive limit | 2-11.8%(V) | | Water Solubility | soluble | | BRN | 1698309 | | InChIKey | ZJCCRDAZUWHFQH-UHFFFAOYSA-N | | LogP | -0.975 (est) | | CAS DataBase Reference | 77-99-6(CAS DataBase Reference) | | NIST Chemistry Reference | 1,3-Propanediol, 2-ethyl-2-(hydroxymethyl)-(77-99-6) | | EPA Substance Registry System | Trimethylolpropane (77-99-6) |
| Safety Statements | 22-24/25 | | WGK Germany | 1 | | RTECS | TY6470000 | | Autoignition Temperature | 375 °C DIN 51794 | | TSCA | Yes | | HS Code | 29054100 | | Hazardous Substances Data | 77-99-6(Hazardous Substances Data) | | Toxicity | LD50 orally in Rabbit: > 2500 mg/kg |
| | Trimethylolpropane Usage And Synthesis |
| Chemical Properties | Trimethylolpropane is a Colorless, hygroscopic crystals. Soluble in water and alcohol. Combustible.The three primary hydroxyl groups undergo the normal OH group reactions. | | Uses | Trimethylolpropane acts as a precursor to alkyd resins, high-gloss coatings and ion exchange resins. It is also employed as a multifunctional monomer utilized for the production of coatings, ethoxylated and propoxylated trimethylolpropane derivatives. Further, it serves as a building block in the polymer industry. | | Application | Trimethylolpropane is used in saturated polyesters for coil coatings, alkyds for paints, polyurethanes for coatings and elastomers, acrylic acid esters for radiation curing, esters for synthetic lubricants, rosin esters and for surface treatment of pigments.
Large quantities of trimethylolpropane and its ethoxylated derivatives are used as precursors for urethanes and polyester resins. Another important field of application is in medium-oil and short-oil alkyd resins (→ Alkyd Resins). The resulting lacquers are characterized by excellent resistance to alkali, detergents, and water, combined with outstanding impact resistance and flexibility, as well as excellent clearness and clearness retention. | | Definition | ChEBI: Trimethylolpropane is a primary alcohol. It is used as a precursor for the manufacture of resins including alkyds, saturated polyesters and polyurethanes (polyester polyol and polycarbonate diol). | | Synthesis | Trimethylolpropane is made by the base-catalyzed aldol addition of butyraldehyde with formaldehyde followed by Cannizzaro reaction of the intermediate 2,2-bis(hydroxymethyl) butanal with additional formaldehyde and at least a stoichiometric quantity of base.
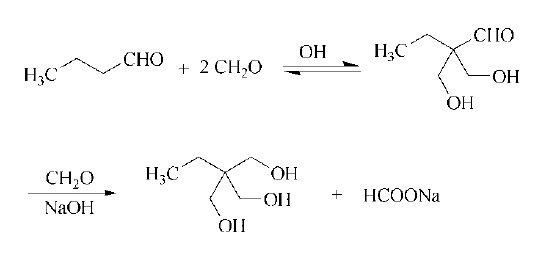
| | Purification Methods | Crystallise it from acetone and ether and it distils at high vacuum. [Beilstein 1 III 2349.] |
| | Trimethylolpropane Preparation Products And Raw materials |
|



