|
| | Atazanavir sulfate Basic information |
| Product Name: | Atazanavir sulfate | | Synonyms: | N-[(2S)-1-[2-[(2S,3S)-2-hydroxy-3-[[(2S)-2-(methoxycarbonylamino)-3,3-dimethyl-1-oxobutyl]amino]-4-phenylbutyl]-2-[(4-phenylphenyl)methyl]hydrazinyl]-3,3-dimethyl-1-oxobutan-2-yl]carbamic acid methyl;BMS 232632 sulfate;BMS 232632 SULFATE;BMS-232632 SULFATE;BMS232632 SULFATE;BMS232632 sulfate;BMS-232632 sulfate;1-(4-Biphenylyl)-4(S)-hydroxy-5(S)-2,5-bis{[N-(methoxycarbonyl-)-L-tert-leucinyl]amino}-6-phenyl-2-azahexane;Aids060276;Aids-060276 | | CAS: | 229975-97-7 | | MF: | C38H54N6O11S | | MW: | 802.94 | | EINECS: | 620-495-2 | | Product Categories: | Inhibitors;peptides;Antiviral Agents;Atazanavir;Inhibitor;BMS-232632-05, Reyataz;229975-97-7 | | Mol File: | 229975-97-7.mol |  |
| | Atazanavir sulfate Chemical Properties |
| Melting point | 195.0°, or acetone; mp 198-199° (dec) | | alpha | D22 -46.1° (c = 1 in 1:1 CH3OH/H2O, pH = 2.6) | | storage temp. | under inert gas (nitrogen or Argon) at 2-8°C | | solubility | ≥28.7 mg/mL in DMSO with gentle warming; insoluble in H2O; ≥4.05 mg/mL in EtOH with gentle warming and ultrasonic | | form | Powder |
| Safety Statements | 24/25 | | HS Code | 29333990 |
| | Atazanavir sulfate Usage And Synthesis |
| Description | Atazanavir (BMS-232632, III), an azapeptide HIV
protease inhibitor, has been developed and launched by
Bristol-Myers Squibb (BMS), under worldwide license from
Novartis, for the treatment of HIV infection. Atazanavir
was launched in the US as Reyataz™ in July 2003. | | Chemical Properties | Off-White Solid | | Uses | Atazanavir is a novel azapeptide HIV protease inhibitor (PI). Antiviral. | | Uses | Atazanavir is a HIV protease inhibitor with Ki of 2.66 nM | | Uses | Atazanavir Sulfate is an intermediate of Atazanavir(A790051) which is a novel azapeptide HIV protease Inhbitor. Antiviral. | | Brand name | Reyataz
(Bristol-Myers Squibb). | | Synthesis | The
synthesis of atazanavir (III) appeared in several reports. The synthetic route depicted in the scheme was one of
the best routes which was suitable for large scale production. The commercially available chiral diol 25 was
converted to its silyl mesylate 26 in one pot via selective
silylation and subsequent mesylation. This oily intermediate
26 was carried into the next step without further purification.
The desilylation of 26 was achieved by using inexpensive
ammonium fluoride. The resulting solid product 27 was
readily isolated and further purified through recrystallization
from IPA/H2O in 80% yield. The epoxide formation from
27 was affected by KOtBu in THF/IPA to provide enantiomerically pure epoxide 28 in 88% yield. Suzuki
coupling of boronic acid 29 with bromopyridine (30)
provided pyridyl benzaldehyde 31 in 80% yield after
crystallization. The subsequent condensation of aldehyde 31
with t-butylcarbamate was carried out by refluxing in
toluene/IPA and Shiff base 32 was collected by filtration
upon cooling. Reduction of hydrazone 32 to hydrazine 33
was accomplished by employing a catalytic phase-transfer
hydrogenation protocol (Pd/C, HCOONa) to furnish
hydrazine 33 in 78% yield after crystallization. Coupling of
the hydrazinocarbamate 33 with epoxide 28 was performed
in refluxing IPA, followed by the addition of water to
precipitate the crude product. Subsequent recrystallization
from MeCN/H2O furnished 34 in 85% yield. Treatment of
34 with concentrated HCl in THF at 50oC removed the two
Boc groups in 34 to give the product as an oil, which was
then dissolved in a mixture of DCM/DIPEA and slowly
transferred into a premixed solution of N-methoxycarbonyl-
L-tert-leucine (35), HOBT, and WSC in DCM. After
removal of the solvent the crude product was crystallized
from IPA/EtOH to furnish the freebase 36 in 82% yield. The
sulfate III was obtained by stirring the free base 36 with
concentrated H2SO4 in EtOH at ambient temperature. Direct
crystallization by addition of n-heptane provided the sulfate
salt III as an easily filterable solid in 85% yield. 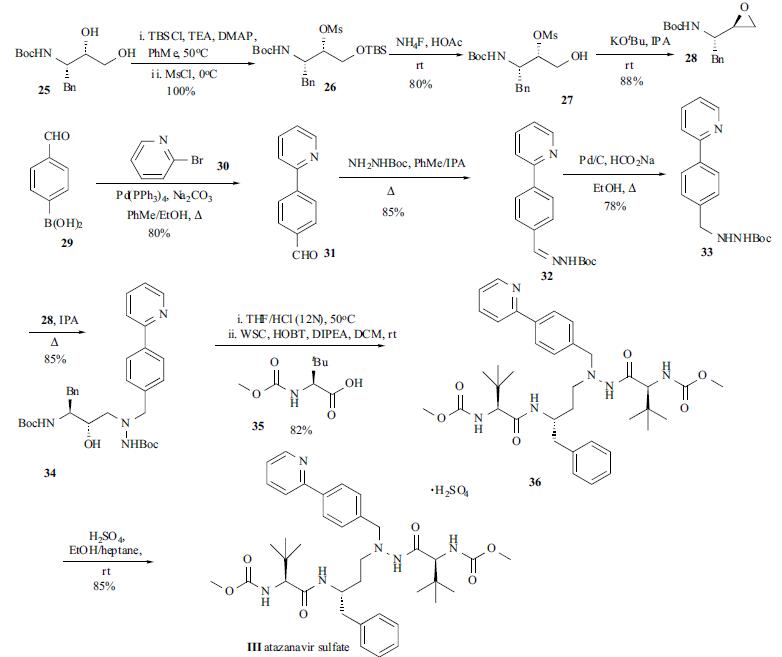
|
| | Atazanavir sulfate Preparation Products And Raw materials |
|