|
| | POLYTHIAZIDE (200 MG) Basic information |
| Product Name: | POLYTHIAZIDE (200 MG) | | Synonyms: | 2h-1,2,4-benzothiadiazine-7-sulfonamide,6-chloro-3,4-dihydro-2-methyl-3-(((2,2;2h-benzo-1,2,4-thiadiazine1,1-dioxide;2-methyl-3-(beta,beta,beta-trifluoroethylthiomethyl)-6-chloro-7-sulfamyl-3,4-d;6-Chloro-2-methyl-3-([(2,2,2-trifluoroethyl)sulfanyl]methyl)-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide;6-Chloro-3,4-dihydro-2-methyl-3-(((2,2,2-trifluoroethyl)thio)methyl)-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide;6-Chloro-3,4-dihydro-2-methyl-3-[[(2,2,2-trifluoromethyl)thio]methyl]-2H-1,2,4-benzothiadiazine -7-sulfonamide 1,1-dioxide;benzothiadiazine-7-sulfonamide1,1-dioxide;Drenusil | | CAS: | 346-18-9 | | MF: | C11H13ClF3N3O4S3 | | MW: | 439.8818296 | | EINECS: | 2064684 | | Product Categories: | | | Mol File: | 346-18-9.mol |  |
| | POLYTHIAZIDE (200 MG) Chemical Properties |
| Melting point | 202.5° | | Boiling point | 580.1±60.0 °C(Predicted) | | density | 1.8346 (rough estimate) | | refractive index | 1.6100 (estimate) | | storage temp. | Refrigerator | | solubility | DMSO (Slightly), Methanol (Slightly, Heated) | | pka | pKa 9.58(H2O,t =25) (Uncertain) | | form | Solid | | color | White to Off-White | | EPA Substance Registry System | Polythiazide (346-18-9) |
| | POLYTHIAZIDE (200 MG) Usage And Synthesis |
| Description | Polythiazide exhibits a more pronounced antihypertensive effect than chlorothiazide and it
may be used independently for the same indications as the aforementioned drugs.
However, it is primarily used as an ingredient of a combination drugs intended for lowering
pressure, in particular in minizide, which is a combination of prazozine and polythiazide. | | Originator | Renese ,Pfizer, US ,1961 | | Uses | Polythiazide is a thiazide diuretic. Polythiazide is used in the treatment of hypertension as well as nephrotic syndrome, cirrhosis of the liver, and nutritional edema. | | Definition | ChEBI: Polythiazide is a benzothiadiazine. | | Manufacturing Process | (A) Preparation of trifluoroethylthioacetaldehyde dimethylacetal: To 4.6 g (0.2 mol) of metallic sodium dissolved in 75 ml of absolute methanol is rapidly added 24.4 g (0.2 mol) of mercaptoacetaldehyde dimethylacetal followed by dropwise addition of 42.0 g (0.2 mol) of trifluoroethyl iodide.
The resulting reddish mixture is refluxed on a steam bath for one hour. One half of the alcohol is removed by concentration and the remainder diluted with several volumes of water and extracted with ether. The combined ether extracts are dried over sodium sulfate, the ether then removed at reduced pressure and the residue distilled to about 30 g (BP 82°C/25 mm).
(B) Preparation of 4-Amino-2-Chloro-5-(Methylsulfamyl)Benzenesulfonamide: The 5-substituted-2,4-disulfamyl anilines may be prepared by procedures described in the literature, for example, the general procedures in Monatsch. Chem. vol. 48, p 87 (1927), which involves the treatment of a m-substituted aniline with from 10 to 20 parts by weight of chlorosulfonic acid followed by the gradual addition of from about 90 to 170 parts by weight of sodium chloride. The resultant mixture is heated at approximately 150°C for about 2hours after which the reaction mixture is poured into water and the resultant 5substituted aniline-2,4-disulfonyl chloride is filtered and is then treated with concentrated ammonium hydroxide or suitable amine by standard procedures to obtain the corresponding disulfonamide.
(C) Preparation of 2-Methyl-3-(2,2,2-Trifluoroethyl)Thiomethyl-6-Chloro-7Sulfamyl-3,4-Dihydro-1,2,4-Benzothiadiazine-1,1-Dioxide: To 4.6 g (0.015 mol) of 4-amino-2-chloro5-(methylsulfamyl)benzenesulfonamide in 30 ml of the dimethyl ether of ethylene glycol is added 4.08 g (0.02 mol) of 2,2,2trifluoroethylmercaptoacetaldehyde dimethylacetal followed by 1 ml of ethyl acetate saturated with hydrogen chloride gas. The resulting solution is refluxed for 1.5 hours, cooled and then slowly added to cold water dropwise with stirring. The crude product is filtered, dried and recrystallized from isopropanol (3.2 g), MP 202° to 202.5°C. A second recrystallization from isopropanol raised the MP to 202° to 203°C. | | Brand name | Renese (Pfizer). | | Therapeutic Function | Diuretic | | General Description | Crystals or white powder. | | Air & Water Reactions | Its rate of decomposition in solution increases with an increase in pH. . Insoluble in water. | | Reactivity Profile | POLYTHIAZIDE (200 MG) is a sulfonamide derivative. With strong reducing agents will produce hydrogen sulfide gas. | | Fire Hazard | Flash point data for POLYTHIAZIDE (200 MG) are not available, but POLYTHIAZIDE (200 MG) is probably combustible. | | Synthesis | Polythiazide, 1,1-dioxide 2-methyl-3-(2,2,2-trifluoroethylthiomethyl)-6-
chloro-3,4-dihydro-2H-1,2,4-benzothiadiazin-7-sulfonamide (21.3.8), is also synthesized
by an analogous scheme, which is by condensing 4-aminosulfonyl-5-chloro-2-methylaminosulfonylaniline
(21.3.7) with 2,2,2-trifluoroethylthioacetaldehyde dimethylacetal. 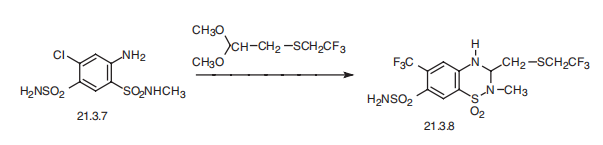
|
| | POLYTHIAZIDE (200 MG) Preparation Products And Raw materials |
|



