|
| | N-Methyldiethanolamine Basic information |
| Product Name: | N-Methyldiethanolamine | | Synonyms: | N,N-BIS(2-HYDROXYETHYL)METHYLAMINE;N-METHYL-2,2'-IMINODIETHANOL;N-METHYL-2,2-IMINOBIS(ETHANOL);N-METHYLDIETHANOLAMINE;N-METHYL-N-(2-HYDROXYETHYL)-2-AMINOETHANOL;METHYL DIETHANOLAMINE;MDEA;BIS(2-HYDROXYETHYL)METHYLAMINE | | CAS: | 105-59-9 | | MF: | C5H13NO2 | | MW: | 119.16 | | EINECS: | 203-312-7 | | Product Categories: | Thanolamine Series | | Mol File: | 105-59-9.mol |  |
| | N-Methyldiethanolamine Chemical Properties |
| Melting point | -21 °C | | Boiling point | 246-248 °C(lit.) | | density | 1.038 g/mL at 25 °C(lit.) | | vapor density | 4 (vs air) | | vapor pressure | 0.01 mm Hg ( 20 °C) | | refractive index | n20/D 1.469(lit.) | | Fp | 260 °F | | storage temp. | Store below +30°C. | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Liquid | | pka | 14.41±0.10(Predicted) | | color | Clear colorless to light yellow | | Odor | Ammonical | | PH | 11.5 (100g/l, H2O, 20℃) | | PH Range | 11.5 at 100 g/l at 20 °C | | explosive limit | 0.9-8.4%(V) | | Water Solubility | MISCIBLE | | BRN | 1734441 | | Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. | | InChIKey | CRVGTESFCCXCTH-UHFFFAOYSA-N | | LogP | -1.16 at 23℃ | | CAS DataBase Reference | 105-59-9(CAS DataBase Reference) | | NIST Chemistry Reference | Methyldiethanolamine(105-59-9) | | EPA Substance Registry System | N-Methyldiethanolamine (105-59-9) |
| Hazard Codes | Xi | | Risk Statements | 36 | | Safety Statements | 24 | | RIDADR | 2735 | | WGK Germany | 1 | | RTECS | KL7525000 | | Autoignition Temperature | 770 °F | | TSCA | Yes | | HS Code | 2922 17 00 | | HazardClass | 8 | | PackingGroup | II | | Hazardous Substances Data | 105-59-9(Hazardous Substances Data) | | Toxicity | LD50 orally in Rabbit: 4680 mg/kg |
| | N-Methyldiethanolamine Usage And Synthesis |
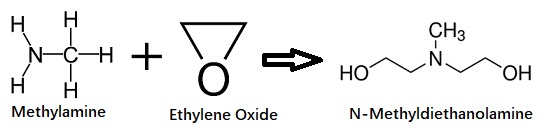
| Chemical Properties | N-Methyldiethanolamine (MDEA) is a colorless to yellow viscous liquid with an ammonia-like odor. It is completely soluble in water. N-Methyldiethanolamine is an alkyl alkanolamine.It combines the chemical characteristics of both amines and alcohols so that it is capable of undergoing reactions typical of both alcohols and amines: forming quaternary amine salts, soaps, and esters. | | Uses | N-methyldiethanolamine is used as an intermediate in the synthesis of numerous products. Its unique chemistry has resulted in its use in diverse areas, including coatings, textile lubricants, polishes, detergents, pesticides, personal-care products, pharmaceuticals, urethane catalysts, and water-treatment chemicals. It is also used in absorption of acidic gases, catalyst for polyurethane foams, pH control agent. | | Uses | N-Methyldiethanolamine is a reagent used for protection of boronic acids as N-methyl-O,O-diethanolamine esters. | | Preparation | N-methyldiethanolamine is produced by the reaction between ethylene oxide and methylamine.
 | | Synthesis Reference(s) | Journal of the American Chemical Society, 107, p. 6659, 1985 DOI: 10.1021/ja00309a039 | | General Description | Colorless liquid. | | Air & Water Reactions | Very water soluble . | | Reactivity Profile | N-Methyldiethanolamine is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. N-Methyldiethanolamine may react with oxidizing materials. | | Health Hazard | Exposure can cause irritation of eyes, nose and throat. | | Fire Hazard | Special Hazards of Combustion Products: Irritating vapors and toxic gases, such as nitrogen oxides and carbon monoxide, may be formed when involved in fire. | | Flammability and Explosibility | Nonflammable | | Environmental Fate | N-methyldiethanolamine is readily biodegradable, and its bioconcentration potential is low. Releases to the environment would not persist and would degrade rapidly. The potential for mobility in soil is very high. It is slightly toxic to aquatic organisms on an acute basis. | | storage | N-methyldiethanolamine is stable under normal storage and use conditions, but elevated temperatures can cause it to decompose. Decomposition products depend upon temperature, air supply, and the presence of other materials. Avoid contact with nitrites, strong acids, and strong oxidizers. Avoid unintended contact with halogenated hydrocarbons. N-methyldiethanolamine can react with halogenated organics, resulting in temperature and/or pressure increases. It is corrosive when wet. Heating above 60°C (140°F) in the presence of aluminum can result in corrosion and generation of flammable hydrogen gas. |
| | N-Methyldiethanolamine Preparation Products And Raw materials |
|



