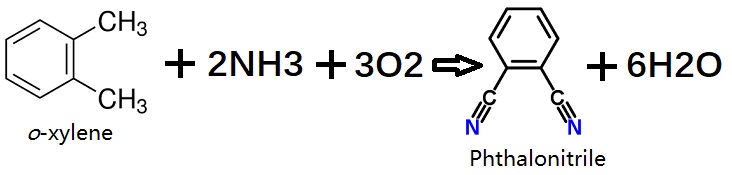|
| | Phthalonitrile Basic information |
| | Phthalonitrile Chemical Properties |
| Melting point | 137-139 °C (lit.) | | Boiling point | 227.54°C (rough estimate) | | density | 1.24 | | vapor pressure | <1 Pa (25 °C) | | refractive index | 1.6231 (estimate) | | Fp | 162°C | | storage temp. | Store below +30°C. | | solubility | benzene: 50 mg/mL, clear | | form | Crystals or Crystalline Powder | | color | White to beige | | PH | 7 (H2O) | | Water Solubility | 0.56 g/L (25 ºC) | | BRN | 775028 | | Exposure limits | ACGIH: TWA 1 mg/m3 | | Stability: | Stable. Incompatible with strong bases, strong acids, strong oxidizing agents, strong reducing agents. | | CAS DataBase Reference | 91-15-6(CAS DataBase Reference) | | NIST Chemistry Reference | Phthalonitrile(91-15-6) | | EPA Substance Registry System | 1,2-Benzenedicarbonitrile (91-15-6) |
| Hazard Codes | T | | Risk Statements | 25-23/24/25 | | Safety Statements | 36/37/39-45-28A | | RIDADR | UN 3439 6.1/PG 2 | | WGK Germany | 1 | | RTECS | TI8575000 | | Autoignition Temperature | >580 °C | | TSCA | Yes | | HazardClass | 6.1 | | PackingGroup | II | | HS Code | 29269095 | | Hazardous Substances Data | 91-15-6(Hazardous Substances Data) | | Toxicity | LD50 orally in Rabbit: 85 mg/kg |
| | Phthalonitrile Usage And Synthesis |
| Chemical Properties | Phthalonitrile is a crystalline powder having a faint grayish yellow color and a slighty aromatic odor, similar to benzonitrile. Phthalonitrile was first described in 1896 when it was isolated during the diazotization of 2-aminobenzonitrile. The compound is sparingly soluble in water (ca. 1 g/L) and soluble in acetone, nitrobenzene, and benzonitrile. It cannot be distilled and polymerizes if heated above the melting point. It is not explosive and is difficult to ignite, but the dust can explode. | | uses | Phthalonitrile is used as a starting material for producing phthalocyanine pigments (→ Phthalocyanines), fluorescent brightners, and photographic sensitizers. | | Uses | Phthalonitrile is used as an intermediate for chemical synthesis in the production of building blocks for colorants and coatings, life science and agricultural chemicals.?And also used as a precursor to phthalocyanine and other pigments, fluorescent brighteners, and photographic sensitizers. | | Uses | Phthalodinitrile monomers can be polymerized thermally in the presence of small amounts of curing agents into thermosetting polymers. Phthalodinitrile is a precursor to phthalocyanine which is used as a dye or pigment. | | Preparation | Phthalonitrile can be produced from phthalic acid, phthalic anhydride, phthalamide, or phthalimide by reaction with ammonia and elimination of water at 300–500°C in the gas phase in the presence of a catalyst.
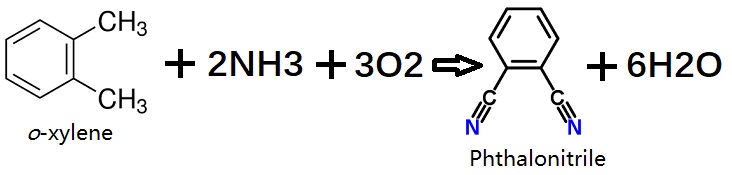
In a single-stage continuous process, o-xylene is converted to phthalonitrile by reaction with ammonia and oxygen in the gas phase in a fluidized-bed reactor. Generally, metal oxide mixtures containing vanadium, antimony, chromium, and molybdenum, with further active components such as iron, tungsten, and alkali-metal oxides, on an alumina or silica support are used as catalysts. | | Reactions | The route from o-phthalodinitrile can be represented 4C8H4N2 +M → MPc, where M is a bivalent metal, metal halide, metal alcoholate, or an equivalent amount of metal of valence other than two in a 4:1 molar ratio. | | Flammability and Explosibility | Notclassified | | Safety Profile | Poison by ingestion,
subcutaneous, and intraperitoneal routes.
Questionable carcinogen with experimental
tumorigenic data. When heated to
decomposition it emits toxic fumes of CN-
and NOx. See also NITRILES. | | Purification Methods | Crystallise the nitrile from EtOH, toluene or *benzene. It has also been distilled under high vacuum. It is steam volatile. [Beilstein 9 H 815, 9 II 602, 9 III 4199, 9 IV 3268.] |
| | Phthalonitrile Preparation Products And Raw materials |
|