|
| Product Name: | Oxybutynin | | Synonyms: | 4-(diethylaMino)but-2-yn-1-yl 2-cyclohexyl-2-hydroxy-2-phenylacetate;Benzeneacetic acid, a-cyclohexyl-a-hydroxy-,4-(diethylaMino)-2-butyn-1-yl ester;α-cyclohexyl-α-hydroxy-benzeneacetic acid-4-(diethylamino)-2-butyn-1-yl ester;4-diethylamino-2-butynylalpha-phenylcyclohexaneglycolate;alpha-cyclohexyl-alpha-hydroxy-benzeneaceticaci4-(diethylamino)-2-butynyl;alpha-phenyl-cyclohexaneglycolicaci4-(diethylamino)-2-butynylester;OXYBUTININ;(+/-)-OXYBUTYNIN | | CAS: | 5633-20-5 | | MF: | C22H31NO3 | | MW: | 357.49 | | EINECS: | 630-332-7 | | Product Categories: | API | | Mol File: | 5633-20-5.mol |  |
| | Oxybutynin Chemical Properties |
| Boiling point | 494.4±45.0 °C(Predicted) | | density | 1.097±0.06 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Room Temperature | | solubility | ≥ 17.9mg/mL in DMSO | | form | Powder | | pka | 8.04(at 25℃) | | CAS DataBase Reference | 5633-20-5(CAS DataBase Reference) |
| | Oxybutynin Usage And Synthesis |
| Medicine for the treatment of urinary incontinence | Urinary incontinence occurs at any age. According to statistics, about 5-15% of the elderly have different levels of urinary incontinence.In the United States, there are 12 million people with urinary incontinence, and 1 out of every 10 elderly people suffer from urinary incontinence. For the group of over 60 years ole, the female patients are twice as many as the male patients. The cost of non hospitalization is $1 billion ~2 billion a year.The market for urinary incontinence is of great potential. With the aging of our population and the improvement of people's quality of life, the research and development of drug for treatment of urinary incontinence has become an urgent problem.Urinary incontinence is mainly treated with anticholinergic drugs or combined with anticholine / antismooth muscle relaxant, which inhibits acetylcholine receptor and inhibits bladder contraction.Some drugs can still play a significant role in the direct relaxation of smooth muscle. Oxybutynin is the first choice as the third generation drug to treat urinary incontinence.It was first synthesized by the United States in 1973 and listed in 1975.It has gradually replaced the first generation of drugs for the treatment of urinary incontinence represented by atropine, scopolamine and imbrominium bromide and the second generation of drugs for the treatment of urinary incontinence represented by flavoxate. It is widely used in 29 countries in Europe. Its molecular weight is 393.9. It is easy to dissolve in water and acid, insoluble in alkali, and is a racemo mixture with chiral carbon atoms.It is a kind of anticholinergic and anti smooth muscle spasmodic drugs.It has a strong selective effect on the smooth muscle of the urinary and reproductive tract.The oral administration absorbs well with small toxic and side effects.
It is the most commonly used medicine for the treatment of urination, frequency of urine and urinary incontinence.It has a mild anticholinergic effect and a strong anti smooth muscle spasticity effect. Low concentration (10-7M) has anticholinergic effect, which is about 1/5 of atropine. When in high concentration (10-5M), it can relax smooth muscle directly, and has strong selective action on smooth muscle of urinary tract and genital tract. Its smooth muscle relaxation effect is different from that of papaverine or flavoxate by inhibition of phosphodieste. Instead, it works by blocking the Ca2+ internal flow and the effect is about 4~10 times of atropine. Oxybutynin inhibits the bladder contractions and spontaneous contractions of the bladder caused by electric stimulation of the peripheral end of the pelvic nerve in cats. Its effectiveness is 0.5 times that of atropine. Oxybutynin dose will dependently increases bladder volume, filling pressure, and pressure threshold for urination,It can relieve the symptoms caused by detrusor instability and hyperreflexism in the detrusor. It also has a similar effect on the human bladder.Oral administration can increase the volume of bladder in patients with neurogenic bladder and dysmotor neurons.It can also reduce the frequency of urination, urgency and urination of urinary incontinence.This product also has local anesthesia and analgesic effect.At 0.02%, it anaesthetizes the rabbit cornea, which is about 2 times as effective as lidocaine.The analgesic effect is independent of anticholinergic effects without resistance and withdrawal symptoms, to alleviate the patient's pain symptoms. It is helpful for clinical treatment.
| | Tolterodine tartrate | Tolterodine tartrate is a new generation of slow release agent muscarinic receptor (M receptor) antagonist,It is of a high affinity and specificity for M receptor.It has a competitive inhibitory effect on M receptor, which can effectively inhibit detrusor contraction. It has been widely used in clinical practice, and is suitable for the treatment of frequent micturition, urgency or urgency of urinary incontinence symptoms. After oral administration, it is metabolized into 5- hydroxymethyl derivatives through the liver.Its anticholinergic action is similar to that of the parent body.The two competitively inhibits the binding of acetylcholine to its receptor.It inhibits the involuntary contraction of the bladder. The effect or affinity for other receptors and potential cell targets, such as calcium channels, is weak.This product can be absorbed quickly after oral administration, and its absorption rate is more than 77%.The Cmax and AUC of oral administration of 1~4mg is linearly related to the dose.There is no need to adjust the dose for the difference of food, age and sex.About 2.5 hours after oral administration of 2mg, it will reach the peak blood concentration.Cmax is 2.5 2.5μg•L-1, and AUC is 11.8μg•h•L-1. The blood concentration of 5- hydroxymethyl active metabolite (DD01) is very similar to that of this product, Cmax is 2.2μg•L-1 and AUC is 12.1μg•h•L-1. The binding rate to plasma protein is higher, and the free type is only (3.70 + 0.13)%.
The binding rate of DD01 and plasma protein is not high, and the free type is (36 + 4)%.
The ratio of the concentration of this product to its metabolite DD01 in blood and plasma is 0.6 and 0.8, respectively.The distribution volume of this product after the intravenous injection of 1.28mg will be (113 + 26.7) L. This product has extensive first effect in the liver, and the main metabolic pathway involves the oxidation of CYP2D6, which forms the active DD01. Further metabolism is 5- carboxylic acid and N- dealkyl 5- carboxylic acid metabolite,The 2 metabolites recovered in urine account for (51 + 14)% and (29 + 6)% respectively.The elimination half-life of this product (t 1/ 2) is 2~3h, and T 1 / 2 of DD01 is 3~4 H. As for the oral administartion of tolterodine marked with 14C by the healthy volunteers, the excretion rate of urine and feces is 77% and 17%, respectively. The excretion rate of the original tolterodine will not reach 1% of the dosage.5%~14% will be reclaimed with active DD01.Most of the radiated active substances are excreted within 24h after administration.
| | Originator | Ditropan, Marion , US ,1975 | | Uses | Oxybutynin can be used as a pharmaceutical formulation for inhibitin body malodour. | | Uses | Oxybutynin(Ditropan) is an anticholinergic medication used to relieve urinary and bladder difficulties, including frequent urination and inability to control urination (urge incontinence), by decreasing muscle spasms of the bladder.Oxybutynin contains one | | Uses | Oxybutynin is intended for relieving unpleasant symptoms associated with emptying the
intestine or urinary bladder. A | | Definition | ChEBI: Oxybutynin is a racemate comprising equimolar amounts of (R)-oxybutynin and esoxybutynin. An antispasmodic used for the treatment of overactive bladder. It has a role as a muscarinic antagonist, a muscle relaxant, an antispasmodic drug, a parasympatholytic, a calcium channel blocker and a local anaesthetic. It is a tertiary amino compound and a racemate. It contains an esoxybutynin and a (R)-oxybutynin. | | Manufacturing Process | A mixture of 394.2 grams of methyl phenylcyclohexylglycolate and 293.1 grams of 4-diethylamino-2-butynyl acetate was dissolved with warming in 2.6 liters of n-heptane. The solution was heated with stirring to a temperature of 60° to 70°C and 8.0 grams of sodium methoxide were added. The temperature of the mixture was then raised until the solvent began to distill. Distillation was continued at a gradual rate and aliquots of the distillate were successively collected and analyzed for the presence of methyl acetate by measurement of the refractive index. The reaction was completed when methyl acetate no longer distilled, and the refractive index observed was that of pure heptane (nD26 = 1.3855). About 3? hours were required for the reaction to be completed.
The reaction mixture was then allowed to cool to room temperature, washed with water, and extracted with four 165 ml portions of 2 N hydrochloric acid. The aqueous extracts were combined and stirred at room temperature to permit crystallization of the hydrochloride salt of the desired product. Crystallization was completed by cooling the slurry in an ice bath, and the product was collected by filtration, pressed dry, and recrystallized from 750 ml of water. Yield of pure crystalline material, 323 grams.
| | Brand name | Oxytrol (Watson). | | Therapeutic Function | Spasmolytic | | General Description | Oxybutynin, 4-Diethylaminobut- 2-ynyl2-cyclohexyl-2- hydroxy-2-phenyl-ethanoate, (Oxytrol) wasone of the first agents specifically developed to exploit the effectsthat cholinergic blocking agents have on the bladder. Bycompetitively blocking the muscarinic receptors, it has directspasmolytic effects on bladder smooth muscle. This reductionin smooth muscle tone allows for greater volumes of urine tobe stored in the bladder, which results in less urinary incontinence,urgency, and frequency. Oxybutynin acts as a competitiveantagonist on M1, M2, and M3 receptor subtypes. | | Synthesis | Oxybutynin, 4-diethylamino-2-butynylic ester |á-phenylclohexaneglycolic
acid (14.1.35), is synthesized either by a Mannich reaction using propargyl ester of
|á-phenyl-|á-cyclohexaneglycolic acid, paraform and diethylamine, or transesterification of
the methyl ester of |á-phenyl-|á-cyclohexaneglycolic acid using 1-acetoxy-4-diethylamino-
2-butene in the presence of sodium methoxide [26]. 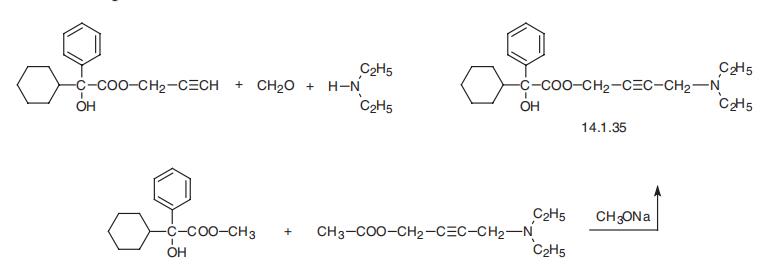
| | Veterinary Drugs and Treatments | Oxybutynin may be useful for the adjunctive therapy of detrusor
hyperreflexi |
| | Oxybutynin Preparation Products And Raw materials |
|



