|
| | Meclozine Basic information |
| Product Name: | Meclozine | | Synonyms: | UCB 5052;UCB 5062;Vomisseis;Vomissels;1-(p-Chloro-a-phenylbenzyl)-4-(m-methylbenzyl)piperazine;mecilizine;NSC 169189;Piperazine, 1-(p-chloro-a-phenylbenzyl)-4-(m-methylbenzyl)- (6CI, 8CI) | | CAS: | 569-65-3 | | MF: | C25H27ClN2 | | MW: | 390.95 | | EINECS: | 209-323-3 | | Product Categories: | | | Mol File: | 569-65-3.mol |  |
| | Meclozine Chemical Properties |
| | Meclozine Usage And Synthesis |
| Originator | Antivert,Roerig,US,1957 | | Uses | Anti-emetic. | | Uses | Meclizine actively affects the vomiting center and is used for vomiting and diarrhea.
Synonyms of this drug are antivert, bonine, lamin, roclizin, and vertol. | | Definition | ChEBI: Meclizine is a diarylmethane. | | Manufacturing Process | 32.3 g of 1-p-chlorobenzhydryl-4-benzyl-piperazine, dissolved in 300 cm3 of alcohol are heated in an autoclave vessel, in the presence of Raney nickel, under a pressure of 100 kg H2, at about 150°C for 6 hours. The catalyst is filtered, the solvent is evaporated and the residue is fractionated under a high vacuum. p-Chlorobenzylhydryl-piperazine (BP 180° to 185°C/1 mm Hg) is isolated with a yield of 75%. Then finely ground NaNH2 is added. The mixture is heated under reflux for 1 hour, the mass is cooled and a molar equivalent of m-methyl benzyl chloride is added.
The solvent is evaporated and the residue is dissolved in chloroform. This
solution is washed with a saturated solution of K2CO3 and dried on K2CO3. The
solvent is evaporated and the residue is distilled under high vacuum. The
product of the condensation distills near 230°C at 2 mm Hg pressure and the
corresponding dihydrochloride melts at 217° to 224°C. | | Brand name | Antivert (Pfizer);Ancoloxine;Bonamina;Bonamine;Calmonal;Chiclida;Cobinamide;Diadril;Dradril;Duremesan;Itinerol;Mecazine;Navicalm;Neo-istafenc;Neo-istafene;Peremesin;Postafene;Premesin;Rovert-m;Ru-vert-m;Sea-leg;Supermesin;Superminal;Suprimal;Taizerl;V-cline;Veritab;Vertizine;Vomaxine;Vomisseis. | | Therapeutic Function | Antinauseant | | World Health Organization (WHO) | Meclozine, an antihistamine with antiemetic activity, was
introduced in 1953 for the treatment of nausea. The action taken in Indonesia in
1963 resulted from concern regarding its possible teratogenic potential.
Subsequent epidemiological studies have been widely accepted, however, as
dispelling this suspicion. Meclozine remains widely available in both prescription
only and over-the-counter preparations and in some countries the licensed
indications include management of nausea of pregnancy. | | Synthesis | Meclizine, 1-[(4-chlorphenyl)methyl]-4-[(3-methylphenyl)phenyl]piperazine
(16.1.16), is synthesized by reductive amination of a mixture of 3-methylbenzaldehyde
with 1-(4-chlorbenzhydryl)piperazine using hydrogen over Raney nickel. 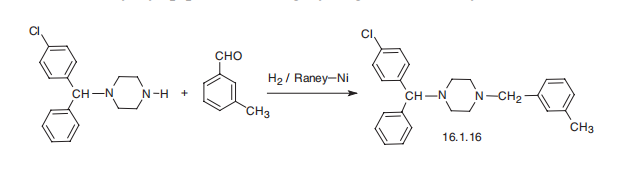
|
| | Meclozine Preparation Products And Raw materials |
|



