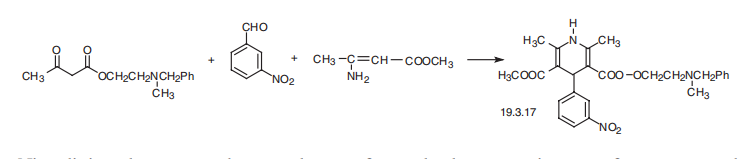|
| | Nicardipine Basic information |
| Product Name: | Nicardipine | | Synonyms: | 1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid methyl 2-[methy(phenyl-methyl)amino]ethyl ester hydrochloride;Nicardipine (base and/or unspecified salts);Methyl 2-(benzyl-methyl-amino)ethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate;1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-[2-[methyl(benzyl)amino]ethyl]5-methyl ester;NicardipineBase;Nicardipine Hcl 54527-84-3 / Base;2-(benzylmethylamino)ethyl methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)pyridine-3,5-dicarboxylate;benzylmethylamino)ethylmethylester | | CAS: | 55985-32-5 | | MF: | C26H29N3O6 | | MW: | 479.52 | | EINECS: | 259-932-3 | | Product Categories: | Active Pharmaceutical Ingredients | | Mol File: | 55985-32-5.mol |  |
| | Nicardipine Chemical Properties |
| Melting point | 136-138 °C | | Boiling point | 603.4±55.0 °C(Predicted) | | density | 1.230±0.06 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | Dichloromethane; Methanol | | pka | 7.30±0.50(Predicted) | | form | Solid | | color | Yellow | | CAS DataBase Reference | 55985-32-5(CAS DataBase Reference) |
| | Nicardipine Usage And Synthesis |
| Originator | Nicodel,Mitsui,Japan,1981 | | Uses | Vasodilator. | | Uses | It is used for arterial hypertension, chronic, stable angina pectoris, preventing angina
pectoris, and for ischemic-type abnormalities of brain blood flow. | | Uses | Nicardipine is a dihydropyridine calcium channel blocker. Antianginal; antihypertensive. Neuroprotective & Neuroresearch products. | | Definition | ChEBI: Nicardipine is a racemate comprising equimolar amounts of (R)- and (S)-nicardipine. It is a calcium channel blocker which is used to treat hypertension. It has a role as an antihypertensive agent, a calcium channel blocker, a vasodilator agent and an autophagy inhibitor. It contains a (S)-nicardipine and a (R)-nicardipine. | | Manufacturing Process | A mixture of 4.98 g of acetoacetic acid N-benzyl-N-methylaminoethyl ester,
2.3 g of β-aminocrotonic acid methyl ester, and 3 g of m-nitrobenzaldehyde
was stirred for 6 hours at 100°C in an oil bath. The reaction mixture was
subjected to a silica gel column chromatography (diameter 4 cm and height
25 cm) and then eluted with a 20:1 mixture of chloroform and acetone. The
effluent containing the subject product was concentrated and checked by thin
layer chromatography. The powdery product thus obtained was dissolved in
acetone and after adjusting the solution with an ethanol solution saturated
with hydrogen chloride to pH 1-2, the solution was concentrated to provide 2
g of 2,6-dimethyl-4-(3'-nitrophenyl)1,4-dihydropyridine-3,5-dicarboxylic acid
3-methylester-5-β-(N-benzyl-N-methylamino)ethyl ester hydrochloride. The
product thus obtained was then crystallized from an acetone mixture, melting
point 136°C to 140°C (decomposed). | | Brand name | Cardene(PDL Biopharma); Cardene (Roche). | | Therapeutic Function | Vasodilator | | Mechanism of action | Nicardipin relaxes smooth musculature of vessels, lowers resistance of coronary and
peripheral vessels, increases blood flow in vessels of the brain, causes a moderate and stable
hypotensive effect, and reduces the myocardial need for oxygen. | | Synthesis | Nicardipine, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-methyl-2-[(methylphenylmethyl)-
amino]ethyl ester 3,5-pirididincarboxylic acid (19.3.7), is synthesized in a
manner analogous to the synthesis of nifedipine, the only difference being that in the
Hantsch synthesis, two different |?-dicarbonyl compounds are used simultaneously with
o-nitrobenzaldehyde. During this, one of these in the enamine form of acetoacetic ester is
simultaneously used as an amine component. A heterocyclization reaction is accomplished
by reacting, the methyl ester of |?-aminocrotonic acid with the 2-methyl-2-benzylaminoethyl
ester of acetoacetic acid. 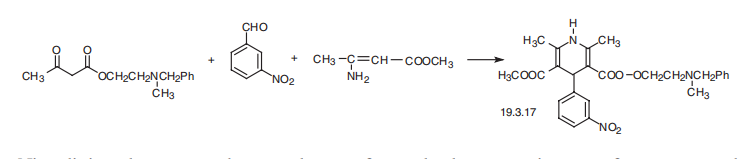
|
| | Nicardipine Preparation Products And Raw materials |
|