|
| | Tadalafil Basic information |
| | Tadalafil Chemical Properties |
| Melting point | 298-300°C | | alpha | D20 +71.0° | | Boiling point | 679.1±55.0 °C(Predicted) | | density | 1.51±0.1 g/cm3(Predicted) | | Fp | 2℃ | | storage temp. | Sealed in dry,2-8°C | | solubility | DMSO: soluble20mg/mL, clear | | pka | 16.68±0.40(Predicted) | | form | powder | | color | white to beige | | optical activity | [α]/D +68 to +78°, c = 1 in chloroform-d | | BCS Class | 4 | | Stability: | Unstable in Methanol | | InChI | InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | | InChIKey | WOXKDUGGOYFFRN-IIBYNOLFSA-N | | SMILES | N1C2=C(C=CC=C2)C2C[C@]3([H])C(=O)N(C)CC(=O)N3[C@H](C3=CC=C4OCOC4=C3)C1=2 | | CAS DataBase Reference | 171596-29-5(CAS DataBase Reference) |
| | Tadalafil Usage And Synthesis |
| Description | Tadalafil (market name “Cialis” or “Adcirca”) was developed by American pharmaceutical company Lilly. It is a kind of PDE5 inhibitor used for the treatment of erectile dysfunction, benign prostatic hypertrophy and pulmonary arterial hypertension.The effect of Tadalafil is relaxing the blood vessels muscles and increasing the blood flow into the corpus cavernosum. Studies show that Cialis works very quickly, taking effect in around 15-20 minutes, and has a prolonged effect that can last for up to 36 hours. T1/2 is 17.5h. | | Chemical Properties | White to Off-White Cyrstalline Solid | | Originator | Lilly/ICOS (US) | | Uses | Tadalafil is used for the treatment of erectile dysfunction. A phosphodiesterase 5 inhibitor. | | Definition | ChEBI: Tadalafil is a pyrazinopyridoindole that is 2,3,6,7,12,12a-hexahydropyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione substituted at position 2 by a methyl group and at position 6 by a 1,3-benzodioxol-5-yl group (the 6R,12aR-diastereomer). A phosphodiesterase V inhibitor inhibitor, currently marketed in pill form for treating erectile dysfunction under the name Cialis; and under the name Adcirca for the treatment of pulmonary arterial hypertension. It has a role as an EC 3.1.4.35 (3',5'-cyclic-GMP phosphodiesterase) inhibitor and a vasodilator agent. It is a pyrazinopyridoindole and a member of benzodioxoles. | | Preparation | Tadalafil is synthesized in three steps starting from D-tryptophan methyl ester, by first condensing with piperonal in a Pictet-Spengler cyclization reaction to form the tetrahydro-β-carboline derivative, which is followed by chloroacetylation of the piperidine ring nitrogen and cyclization with methylamine. | | Brand name | Cialis (Lilly). | | General Description | Tadalafil, 171596-29-5, is a potent PDE5 inhibitor.It received FDA approval for the treatment of erectiledysfunction in December 2003. Because of its half-life of17.5 hours, it is marketed as a 36-hour treatment. Tadalafil ispredominantly metabolized by hepatic enzymes, includingCYP3A4. The concomitant use of CYP3A4 inhibitors suchas ritonavir, indinavir, ketoconazole, as well as moderateCYP3A inhibitors such as erythromycin have been shown toresult in significant increases in tadalafil plasma levels.Much like sildenafil, tadalafil is under clinical investigationfor managing PAH. | | Mechanism of action | The mechanism of action of tadalafil is through inhibiting the activity of the cGMP specific phosphodiesterase type 5 (PDE5). PDE5 degrades cGMP in the corpus cavernosum located around the penis. Therefore, tadalafi leads to the increased concentration of cGMP which further causes the smooth muscle relaxation and increased blood flow into the corpus cavernosum. Some clinical studies also implied that tadalafil could improve endothelia function in men with increased cardiovascular risk and lower the urinary tract symptoms secondary to benign prostatic hyperplasia. | | Pharmacokinetics | Tadalafil is different in structure from both sildenafil and vardenafil. It is rapidly absorbed and peaks in concentration (378 μg/L after a
20-mg dose) after 2 hours, displaying a long half-life of 17.5 hours. It also is metabolized by the liver (CYP3A4). Notably, its
pharmacokinetics is not clinically influenced by alcohol or food intake or by factors such as diabetes or impaired hepatic or renal
function. | | Clinical Use | Tadalafil is one of the two new PDE5 inhibitors launched for the oral treatment of male erectile dysfunction. Tadalafil is a b-carboline derivative and it is structurally distinct from vardenafil (Levitraw) and sildenafil (Viagraw), both of which are PDE5 inhibitors based on a fused pyrimidine core structure. | | Side effects | The most common drug-related adverse events are headache, back pain, dyspepsia, and myalgia. At 10 and 20 mg doses, Tadalafil does not have a significant effect on blood pressure and heart rate and does not result in increased instances of myocardial infarction. Rare reports of prolonged erections greater than 4 h and priapism have been noted with the use of tadalafil. Priapism, if not treated properly, can result in irreversible damage to the erectile tissue. Patients who have an erection lasting greater than 4 h are advised to seek emergency medical attention. | | Synthesis | D-(-)-Tryptophan methyl ester (175) and 1,3-
benzodioxole-5-carboxaldehyde (176) were subjected to a
modified Pictet-Spengler reaction to form cis- and transtetrahydro-
|?-carboline tricyclic compounds. The ciscompound
177 was isolated as a white solid in 42% yield.
The basic nitrogen in the piperidine ring of 177 was acylated
with chloroacetyl chloride (179) to give compound 180 in
93% yield. Finally, the diketonepiperazine ring was formed
by adding 180 to 33% methylamine in ethanol under
refluxing conditions and yielded tadalafil (XXII) in 77% as
a white solid. 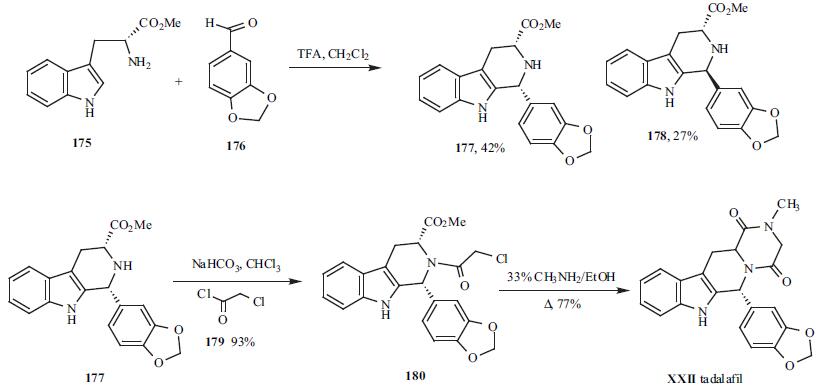
| | Drug interactions | Potentially hazardous interactions with other drugs
Alpha-blockers: enhanced hypotensive effect - avoid
concomitant use.
Antibacterials: concentration possibly increased by
clarithromycin and erythromycin; concentration
reduced by rifampicin - avoid.
Antifungals: concentration increased by ketoconazole
- avoid; concentration possibly increased by
itraconazole.
Antivirals: concentration possibly increased by
fosamprenavir and indinavir; increased by ritonavir -
avoid; increased risk of ventricular arrhythmias with
saquinavir - avoid; avoid high doses of tadalafil with
telaprevir.
Cobicistat: concentration of tadalafil possibly
increased - reduce dose of tadalafil.
Nicorandil: possibly enhanced hypotensive effect -
avoid concomitant use.
Nitrates: enhanced hypotensive effect - avoid
concomitant use.
Riociguat: enhanced hypotensive effect - avoid
concomitant use. | | Metabolism | Tadalafil(171596-29-5) is metabolised in the liver mainly by the cytochrome P450 isoenzyme CYP3A4. The major metabolite, the methylcatechol glucuronide, is inactive. Tadalafil is excreted, mainly as metabolites, in the faeces (61% of the dose), and to a lesser extent the urine (36% of the dose). | | storage | Store at -20°C | | Clinical claims and research | In a study of 348 cases of mild to severe erectile dysfunction, patients were randomly given 20mg of Cialis or a placebo. Results showed that in comparison to the placebo group, patients who took Cialis experienced improved intercourse success in the 24-36 hours following medication, with many patients achieving successful sexual intercourse twice in 36 hours. Side effect rate and severity were also no different from those of the placebo group. Over 5% of patients in the Cialis group experienced headaches and indigestion. | | Precautions | Patients currently taking nitrates, experiencing angina pectoris, suffering from heart disease, patients who have unregulated hypertension or hypotension, or patients who have had a stroke in the past 6 months should not take Cialis. | | References | https://www.drugs.com/tadalafil.html
https://www.drugbank.ca/drugs/DB00820
Roehrborn, C. G., et al. "Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study." Journal of Urology 180.4(2008):1228.
Rosano, Giuseppe M. C., et al. "Chronic Treatment with Tadalafil Improves Endothelial Function in Men with Increased Cardiovascular Risk." European Urology 47.2(2005):214-222.
|
| | Tadalafil Preparation Products And Raw materials |
|



