|
| Product Name: | KP 103 | | Synonyms: | KP 103;KP 103 (pharmaceutical);(2R,3R)-2-(2,4-Difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1,2,4-triazol-1-yl)-2-butanol;Efinaconazole(KP 103);(R,R)-2-(2,4-Difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1,2,4-triazol-1-yl)-2-butanol;(αR,βR)-α-(2,4-Difluorophenyl)-β-methyl-4-methylene-α-(1H-1,2,4-triazol-1-ylmethyl)-1-piperidineethanol;1-Piperidineethanol, α-(2,4-difluorophenyl)-β-methyl-4-methylene-α-(1H-1,2,4-triazol-1-ylmethyl)-, (αR,βR)-;Efinaconazole
(Jublia) | | CAS: | 164650-44-6 | | MF: | C18H22F2N4O | | MW: | 348.39 | | EINECS: | 813-597-5 | | Product Categories: | Inhibitors | | Mol File: | 164650-44-6.mol |  |
| | KP 103 Chemical Properties |
| Melting point | 192-195°C | | Boiling point | 512.2±60.0 °C(Predicted) | | density | 1.26±0.1 g/cm3(Predicted) | | storage temp. | -20°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | pka | 12.11±0.29(Predicted) | | form | powder | | color | white to beige | | optical activity | [α]/D -85 to -95°, c = 1 in chloroform |
| | KP 103 Usage And Synthesis |
| Description | In October 2013, efinaconazole (also known as KP-103) was approved in Canada as a 10% topical solution for the treatment of onychomycosis. Like other azole antifungal agents, efinaconazole acts by disrupting fungal cell membranes through inhibition of sterol 14α-demethylase, an enzyme involved in the biosynthesis of ergosterol, which is a key component of the fungal cell membrane. Efinaconazole has potent antifungal activity against clinical isolates of dermatophytes, including Trichophyton mentagrophyes (MIC80 =0.125 μg/mL) and Trichophyton rubrum (MIC80 =0.25 μg/mL), as well as against Candida and Malassezia species. Unlike other antifungal agents, efinaconazole retains activity in the presence of keratin, indicating that more unbound drug is available at the site of action. Efinaconazole is efficacious in guinea-pig models of fungal infection. Efinaconazole is prepared by reaction of an epoxide intermediate with 4-methylenepiperidine. | | Description | Efinaconazole is a broad-spectrum triazole antifungal agent with activity against Acremonium, Aspergillus, Candida, Cryptococcus, Epidermophyton, Fusarium, Microsporum, Paecilomyces, Pseudallescheria, Scopulariopsis, Trichophyton, and Trichosporon. It inhibits the growth of T. rubrum and T. mentagrophytes clinical isolates with MIC values ranging from ≤2.0 to 60 ng/ml and of C. albicans isolates with MIC values ranging from ≤0.5 to >250 ng/ml. Efinaconazole inhibits sterol 14α-demethylase, which arrests ergosterol biosynthesis at the fungal membrane. It inhibits ergosterol biosynthesis in T. mentagrophytes and C. albicans with IC50 values of 7.0 and 0.40 ng/ml, respectfully. Topical formulations containing efinaconazole have been used for the treatment of onychomycosis. | | Originator | Kaken Pharmaceuticals (Japan) | | Uses | Efinaconazole has been used as:
- a topical anti-onychomycosis drug to determine its effects on Trichophyton rubrum and Trichophyton interdigitale
- as an anti-fungal agent to study its permeability into the nail lysates
- as an anti-fungal agent to study its effects on Candida africana and Candida dubliniensis
| | Definition | ChEBI: A member of the class of triazoles that is butan-2-ol which is substituted at positions 1, 2, and 3 by 1,2,4-triazol-1-yl, 2,4-difluorophenyl, and 4-methylenepiperidin-1-yl groups, respectively (the 2R,3R stereoisomer). It
is an antifungal drug used for the topical treatment of onychomycosis (a nail infection caused mainly by dermatophytes). | | Brand name | Jublia | | Biochem/physiol Actions | Efinaconazole is a triazole antifungal drug approved clinically for the treatment of nail fungus (Onychomycosis). It inhibits sterol biosynthesis by inhibition of cytochrome P450 14α-demethylase, an enzyme in the sterol biosynthesis pathway that leads from lanosterol to ergosterol. Efinaconazole has better nail penetration, so is more effective than other topical agents and as effective as oral medication for nail fungus. | | Synthesis | Commercially available (R)-methyl lactate (55) was first converted
to THP protected alcohol 57 in 4 steps and 78% yield via
morpholino amide 56. Grignard displacement of the morpholine
afforded ketone 58 in 81% yield. Next, ketone 58 was epoxidized
by means of the Corey ylide followed by ring-opening of the epoxide
by triazole which had been activated by exposure to sodium tbutoxide.
Finally, subjection to methanesulfonic acid furnished
diol 59 in 51% yield as the corresponding mesylate salt. Diol 59
was then converted to epoxide 60 through the use of mesyl chloride
and triethylamine in 78% yield and >99% ee. Finally, treatment of epoxide 60 with 4-methylene piperidine¨CHBr in the presence of
lithium hydroxide afforded efinaconazole (VIII) in 87% yield. 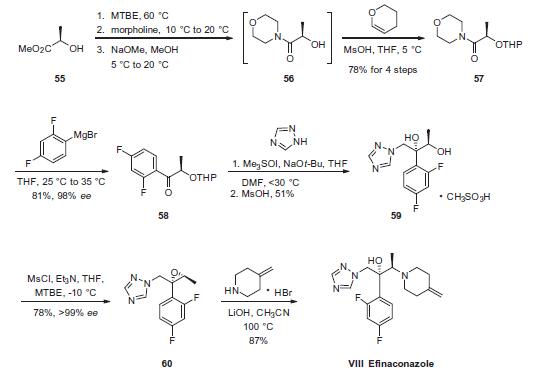
|
| | KP 103 Preparation Products And Raw materials |
|