|
| | N-(Hydroxymethyl)phthalimide Basic information |
| | N-(Hydroxymethyl)phthalimide Chemical Properties |
| Safety Statements | 22-24/25 | | WGK Germany | 3 | | RTECS | TI5270000 | | TSCA | Yes | | HS Code | 29251995 |
| | N-(Hydroxymethyl)phthalimide Usage And Synthesis |
| Chemical Properties | white crystalline powder | | Uses | N-(Hydroxymethyl)phthalimide is used as a reactant in the synthesis of 5-[(3-aralkyl amido/imidoalkyl)phenyl]-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines as antiviral agents. | | Uses | For amidomethylation of aromatics in triflic acid. | | Definition | ChEBI: A primary alcohol comprising phthalimide carrying an N-hydroxymethyl substituent. | | Preparation | To a flask equipped with a mechanical stirrer and condenser is added 511 gm (3.47 moles) of phthalimide, 260 ml of 40% formalin (3.47 moles), and 1750 ml of water. The mixture is refluxed for about 5-10 min or until a clear solution results (if any insoluble material remains, it is first filtered). Then the mixture is cooled for several hours. The resulting product is filtered with suction, washed with cold water, and air-dried to afford 594 gm (96%), m.p. 137-141°C.
NOTE: The product should not be oven-dried since it decomposes with the loss of formaldehyde. Recrystallization of the product from ethanol affords 94% recovery of the original material with the same melting point range.
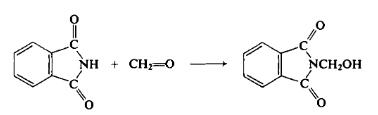
| | Synthesis Reference(s) | Journal of the American Chemical Society, 77, p. 1913, 1955 DOI: 10.1021/ja01612a068 |
| | N-(Hydroxymethyl)phthalimide Preparation Products And Raw materials |
|



