|
| | BIS(TRIMETHYLSILYL) SULFIDE Basic information |
| | BIS(TRIMETHYLSILYL) SULFIDE Chemical Properties |
| Boiling point | 164 °C(lit.) | | density | 0.846 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.4586(lit.) | | Fp | 79 °F | | storage temp. | Flammables area | | solubility | Miscible with terahydrofuran and toluene. | | form | Liquid | | color | Colorless | | Specific Gravity | 0.851 | | Water Solubility | Soluble in tetrahydrofuran and toluene. Hydrolyzes in water. | | Sensitive | Air Sensitive | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | BRN | 1740046 | | Stability: | store cold | | CAS DataBase Reference | 3385-94-2(CAS DataBase Reference) | | EPA Substance Registry System | Disilathiane, hexamethyl- (3385-94-2) |
| Hazard Codes | T | | Risk Statements | 10-23/24/25 | | Safety Statements | 36/37/39-45 | | RIDADR | UN 1993 3/PG 3 | | WGK Germany | 3 | | F | 10-13-21 | | TSCA | Yes | | HazardClass | 3 | | PackingGroup | II | | HS Code | 29319090 |
| | BIS(TRIMETHYLSILYL) SULFIDE Usage And Synthesis |
| Chemical Properties | clear colorless to slightly yellow liquid | | Physical properties | bp 164°C; d 0.846 g cm?3. | | Uses | Bis(trimethylsilyl)sulfide is used as a silylating agent in the synthesis of pseudohalides, trifluoroacetate and tetramethylsilyl halides. It is used in the transformation of oxides and chlorides into corresponding sulfides. It is also used in the preparation of dimethyltrisulfane as well as thiones from aldehydes and ketones. Further, it is used as a reducing agent to reduce aromatic nitro compounds to amines. | | Uses | Hexamethyldisilathiane may be used in the bis-O-demethylation of dimethoxy aromatic compounds.
It may also be used as a sulfur source in the following conversion:
- Amides and lactams to their corresponding sulfur analogs.
- Allyl alcohols to diallyl sulfides.
- Transition metal halides to metal sulfides.
- Aryl iodides to diaryl sulfides.
| | Uses | Bis(trimethylsilyl) sulfide (TMS2S) is used
to reduce aromatic nitro groups to amines and the oxides of sulfur,
selenium, and tellurium. Conditions for nitro group reduction
(eq 1) are forcing; however, yields are good. Reactions of TMS2S
with primary aliphatic nitro groups result in the formation of
the thiohydroxamic acids (eq 2) which can be isolated or carried
further to the nitrile. Secondary alkyl nitro derivatives provide oximes. Sulfoxides are reduced to sulfides, selenoxides to selenides,
and telluroxides to tellurides. Conditions are mild and
work well on both the aliphatic and aromatic oxides.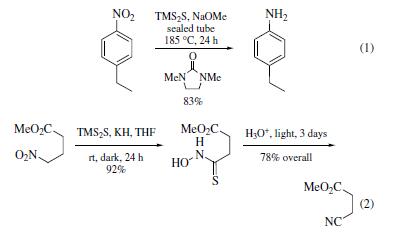 | | Purification Methods | Dissolve it in pet ether (b ca 40o), remove the solvent and distil it. Redistil it under atmospheric pressure of dry N2. It is collected as a colourless liquid which solidifies to a white solid in Dry-ice. On standing for several days it turns yellow possibly due to liberation of sulfur. Store it below 4o under dry N2. [Eaborn J Chem Soc 3077 1950, Beilstein 4 IV 4033.] |
| | BIS(TRIMETHYLSILYL) SULFIDE Preparation Products And Raw materials |
|