|
| | POLYISOPRENE Basic information |
| | POLYISOPRENE Chemical Properties |
| | POLYISOPRENE Usage And Synthesis |
| Description | Polyisoprene is the polymer known as natural rubber, although it can also be manufactured. The natural rubber latex is harvested from the rubber tree, Hevea brasiliensis. This substance has a variety of natural additives, such as proteins and sugars. The polymer from the natural latex is resistant to many solvents and also is easily processed. The synthetic form of this rubber is produced from a pure isoprene solution with a stereospecific isomer to produce the more commonly used cis-l,4 isomer. These rubbers are resistant to abrasion and most solvents and are commercially used in automobile tires, adhesives, and a variety of products that come in close contact with the general public. Their use in baby bottle nipples is a good indication of the extremely low toxicity associated with these elastomers.
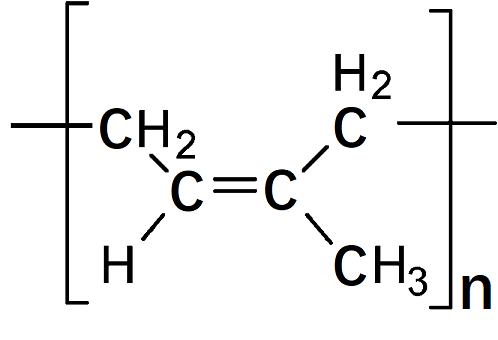
| | Chemical Properties | There are two main solvents for rubber: turpentine and
naptha (petroleum). Because rubber does not dissolve easily,
the material is finely divided by shredding prior to immersion.
Natural rubber has been partially replaced by synthetics,
particularly styrene–butadiene, as a generalpurpose
rubber. High resilience, low heat buildup, and
easy processing are particular advantages of natural rubber
when it is often used in blends with synthetic polyisoprene
and other elastomers. Natural rubber, alone and in combination
with neoprene, has been rated highly for resistance to
water, dimethyl sulfoxide, and some alcohols in a comparative
test of glove materials; resistance to other solvents varied
from good to poor. Polyisoprene supports combustion. | | Chemical Properties | Natural rubber is the name applied to the polymer cis-1,4-polyisoprene obtained chiefly from the 4590 RUBBER, NATURAL latex of the Hevea brasiliensis tree. | | Uses | Natural rubber is a vital, strategic, and irreplaceable raw material used in enormous quantities by the commercial, medical, transportation, and defense industries. At least 40,000 different products and over 400 medical devices contain natural rubber. | | Definition | The major component of natural rubber, also made synthetically. Forms are stereospecific cis-1,4and trans-1,4-polyisoprene.Both can be produced synthetically by the effect of heat and pressure on isoprene in the presence of stereospecific catalysts. Nat | | Production Methods | The latex of natural rubber is obtained from trees (Hevea
brasiliensis); the actual monomer is isopentenyl pyrophosphate
that has been formed by biosynthesis. Natural rubber
contains low-molecular-weight impurities; small amounts of
sugar, fatty acids, proteins, and trace metals all play an
important part in processing.
Depending on the catalyst, rubber may undergo 1,2-; 3,4-;
or 1,4- additional polymerization that leads to several isomeric
structures.
Almost all commercial synthetic polyisoprenes are prepared
from purified isoprene monomer by a solution process.
A stereospecific catalyst, such as an Al–Ti Ziegler type, is
required for direct polymerization to the cis-1,4 isomer.
The production of the finished polymer requires two
separate manufacturing processes: (a) formation of the
rawpolymer and (b) conversion of the polymer to the finished
rubber product. The first step is similar to that of plastic
production. Large-scale operations use bulk materials in an
enclosed system. | | General Description | Available as part of Negative Photoresist kit 654892 | | Industrial uses | Rubber is characterized as being a highly elastic or resilient material, and the natural product is obtained mainly as a latex from cuts in the trunks of the Hevea brasiliensis tree. The latex consists of small particles (averaging about 2500 ? units in diameter) of rubber suspended in an aqueous medium (at about 35% solids content). The system also contains about 6 to 8% nonrubber constituents, some of which are emulsifiers, naturally occurring antioxidants, and proteins.
Natural rubber is used for making many types of articles. Because of its abrasion-resistant quality and low hysteresis in reinforced compounds, it is used in truck-tire tread stocks and in conveyor belts that which are employed in conveying abrasive material such as coal, crushed rock, ore, and cinders. In large tires, it has found application in carcass compounds because of the tack and building qualities of the raw polymer. It has also been used in carcass compounds because of the low heat buildup (low hysteresis) of the carcass compound vulcanizate during severe service conditions in tire usage. |
| | POLYISOPRENE Preparation Products And Raw materials |
|