|
| | Ninhydrin hydrate Basic information |
| Product Name: | Ninhydrin hydrate | | Synonyms: | 1,2,3-Indantrione monohydrate, 2,2-Dihydroxy-1,3-indanedione, Trioxohydrindene monohydrate;Ninhydrin , (1,2,3-Indantrione monohydrate);Ninhydrin (Ethanol Solution) (contains Acetic Acid) [for TLC Stain];Ninhydrin, Monohydrate, Reagent;DIHYDROXYHYDRIN-1,3-INDANDIONE;INDANE-1,2,3-TRIONE HYDRATE;INDANETRIONE HYDRATE;INDANTRIONE HYDRATE | | CAS: | 485-47-2 | | MF: | C9H6O4 | | MW: | 178.14 | | EINECS: | 207-618-1 | | Product Categories: | Organic Building Blocks;Pyridines ,Halogenated Heterocycles;Analytical Chemistry;TLC Stains;Building Blocks;C9;Carbonyl Compounds;Chemical Synthesis;Ketones | | Mol File: | 485-47-2.mol |  |
| | Ninhydrin hydrate Chemical Properties |
| Melting point | 250 °C (dec.) (lit.) | | Boiling point | 230.35°C (rough estimate) | | density | 0.862 | | vapor density | 6.16 (vs air) | | refractive index | 1.4209 (estimate) | | Fp | 13 °C | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | Soluble in water (50 mg/ml,with sonication), ethanol (100 mg/ml), chloroform (Slightly), ether (Slightly), and methanol (100 mg/ml). | | pka | 8.47±0.20(Predicted) | | form | Powder/Solid | | color | white to brownish-white, crystals | | Specific Gravity | 0.788 | | Odor | Odorless | | PH Range | 4.6 - 5.0 | | PH | 4.6-5.0 (10g/l, H2O, 20℃) | | Water Solubility | 0.1-0.5 g/100 mL at 20 ºC | | Sensitive | Light Sensitive | | Merck | 14,6554 | | BRN | 1910963 | | Stability: | Stable. Incompatible with amines, alkalies, strong bases. Light-sensitive. | | InChIKey | FEMOMIGRRWSMCU-UHFFFAOYSA-N | | LogP | 0.670 | | CAS DataBase Reference | 485-47-2(CAS DataBase Reference) | | NIST Chemistry Reference | Ninhydrin(485-47-2) | | EPA Substance Registry System | Ninhydrin (485-47-2) |
| | Ninhydrin hydrate Usage And Synthesis |
| Description | Ninhydrin is historically the most common reagent for processing fingerprints on porous substrates. It has been used as a fingerprint reagent since the 1950s but has been used as a cell stain since the early 1900s. Ninhydrin molecules form a complex with amino acids resulting in a purple product known as Ruhmann's purple. Purple fingerprints are easily visible on light-colored porous substrates but may be difficult to see on patterned or dark-colored substrates. DFO reacts with amino acids to produce a fluorescent product that is not readily seen with the naked eye. This makes it a useful reagent on dark or patterned porous items. DFO prints are viewed with an alternate light source or laser set to 530–570 nm (green light) through a red barrier filter. 1,2-indanedione is an emerging fingerprint reagent that results in visible pink fingerprints that are also fluorescent. The fluorescence is viewed under the same conditions as DFO.
These chemicals can be used on their own or in sequence to develop latent prints on porous substrates. If they are used in sequence, they are used in the following order: indanedione, DFO, ninhydrin. If a reagent is used out of sequence, it will inhibit or limit the effectiveness of the other reagents. In this laboratory exercise you will develop fingerprints on a porous substrate using indanedione, DFO, and ninhydrin.
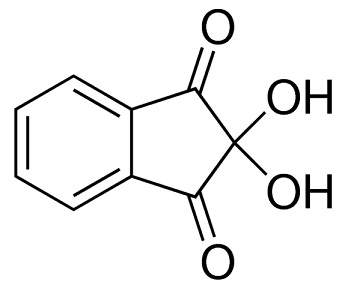
ninhydrin structure
| | Chemical Properties | White to pale yellow crystalline powder | | History | Ninhydrin was discovered in 1910 by the German-English chemist Siegfried Ruhemann (1859–1943). In the same year, Ruhemann observed ninhydrin's reaction with amino acids. In 1954, Swedish investigators Oden and von Hofsten proposed that ninhydrin could be used to develop latent fingerprints. | | Uses | Ninhydrin is used for the detection of free amino groups in amino acids, peptides and proteins. | | Uses | Ninhydrin is an amino acid-sensitive reagent for the development of latent fingermarks on porous surfaces. More sensitive alternatives, such as DFO and IND-Zn, are now available and these have advantages on porous substrates that are not highly luminescent. However, ninhydrin can still be applied after these treatments and may reveal additional ridge detail, particularly on highly luminescent substrates where luminescence visualization may be problematic. | | Definition | ChEBI: A member of the class of indanones that is indane-1,3-dione bearing two additional hydroxy substituents at position 2. | | Reactions | Ninhydrin reacts with primary and secondary amines (including amino acids, proteins, and peptides) to give a dark purple product known as Ruhemann's purple (RP) . As the eccrine component of a latent mark deposit contains amino acids, this reaction can be exploited as a means of developing fingermarks on porous surfaces such as paper and cardboard. The use of ninhydrin as a fingermark detection reagent was first proposed in 1954 by Odén and von Hofsten (1954). Since then, ninhydrin has become the most popular technique for fingermark detection on porous substrates.
Eccrine glands secrete a range of different amino acids that may ultimately be present in a latent fingermark deposit (Hamilton 1965; Ramotowski 2001). Ninhydrin is a nonspecific amino acid reagent in that it reacts in the same manner with different amino acids. In this way, each amino acid present in the latent fingermark deposit will contribute to the developed fingermark image. Amino acids are stable compounds that, due to an affinity for cellulose, do not migrate to any significant extent through dry paper substrates. As a result, very old latent marks can be developed with ninhydrin (the development of 40-year-old marks has been recorded), and the revealed marks are normally of good quality. In addition, the amino acid composition of the eccrine secretion appears to remain relatively constant. Due to these qualities, the use of amino acid reagents (ninhydrin and ninhydrin analogs, including 1,8-diazafluoren-9-one [DFO]) constitutes an effective chemical technique for the development of latent fingermarks on paper surfaces. | | Synthesis Reference(s) | Synthetic Communications, 24, p. 695, 1994 DOI: 10.1080/00397919408012648
Tetrahedron Letters, 9, p. 6201, 1968 | | General Description | White to light yellow crystals or powder. Becomes anhydrous with reddening at 257-266°F. | | Air & Water Reactions | Slightly soluble in water. | | Reactivity Profile | Ninhydrin hydrate is sensitive to prolonged exposure to light. Ninhydrin hydrate yields highly fluorescent ternary compounds with aldehydes and primary amines. | | Hazard | Irritant. | | Health Hazard | Symptoms of exposure to this compound may include skin irritation and
sensitization, and redness of the skin.
ACUTE/CHRONIC HAZARDS: This
compound causes irritation on contact. When heated to decomposition it
emits acrid smoke and fumes. | | Fire Hazard | Flash point data for Ninhydrin hydrate are not available. Ninhydrin hydrate is probably combustible. | | Synthesis | The synthesis of Ninhydrin hydrate is as follows:
A sealed-pressurised reaction vessel (5mL) equipped with a magnetic stirrer was charged with indan-1-one (1equiv), selenium dioxide (3.1equiv) and dioxane/water (3mL/0.3mL). It was then irradiated in a Biotage Initiator Microwave synthesizer 2.0 440W with microwave heating to 180°C with a maximum of 400W for 5min. Then, the vessel was rapidly forced-air cooled to room temperature. The mixture was transferred into a round bottom flask, and the vessel washed with acetone. Silica was added to prepare a solid deposit. The volatile solvents were then evaporated in vacuo before purification by flash chromatography (ethyl acetate/cyclohexane) to afford the corresponding ninhydrin.
 | | Purification Methods | Crystallise ninhydrin from hot water (charcoal). Dry it under vacuum and store it in sealed brown containers. [Beilstein 7 IV 2786.] |
| | Ninhydrin hydrate Preparation Products And Raw materials |
|




