|
| | POLY(VINYL METHYL ETHER) Basic information |
| | POLY(VINYL METHYL ETHER) Chemical Properties |
| Melting point | 112-144 °C | | density | 1.03 g/mL at 25 °C | | vapor pressure | 0.03 atm ( 20 °C) | | refractive index | n20/D 1.467 | | Water Solubility | Soluble in water | | form | clear liquid | | color | Colorless to Yellow to Orange | | CAS DataBase Reference | 9003-09-2 | | EPA Substance Registry System | Ethene, methoxy-, homopolymer (9003-09-2) |
| Risk Statements | 36/37/38 | | Safety Statements | 26-36/37/39 | | RIDADR | UN 1230 3/6.1/PG II | | WGK Germany | 1 | | HS Code | 2909.19.6000 | | HazardClass | 3/6.1 | | PackingGroup | II |
| | POLY(VINYL METHYL ETHER) Usage And Synthesis |
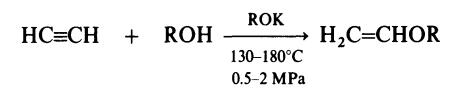
| Definition | ChEBI: A macromolecule composed of repeating methoxyethyl groups. | | Preparation | Vinyl ethers are prepared by reaction of acetylene and alcohols in the
presence of the potassium alkoxide as catalyst:
For the production of polymers, methyl, ethyl and isobutyl vinyl ethers are
the most common monomers.
Vinyl ethers are susceptible only to cationic polymerization. In a typical process, methyl vinyl ether is agitated with boron
trifluoride (BF 3. 2H20). The temperature is kept at about l0?? by cooling
for 3-4 hours; the reactor is then sealed and the temperature allowed to rise
to 100??C. Reaction is complete in about 10 hours and the polymer is obtained
as a viscous mass. It is interesting to note that poly(vinyl isobuty ether) may
be obtained in crystalline form by conducting polymerization at - 80?? to
-60??C in liquid propane using boron trifluoride etherate as initiator. This
was the first stereoregular polymerization to be achieved. Incidentally,
isotactic polymer has also been prepared by the use of Ziegler-Natta type
catalysts and this was the first indication that these catalysts could polymerize a suitable monomer by a cationic mechanism.
Poly(vinyl methyl ether) is water-soluble and is used in adhesive and textile sizes. The ethyl and isobutyl polymers find use in pressure-sensitive adhesives. |
| | POLY(VINYL METHYL ETHER) Preparation Products And Raw materials |
|