|
| | 8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione Basic information |
| Product Name: | 8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione | | Synonyms: | Itraphylline;Istradefylline-13C-d3;CS-285;8-[(1E)-2-(2-(3,4-Dimethoxyphenyl)ethenyl]-1,3-diethyl-3,7-dihydro-7-methyl-1H-purine-2,6-dione;8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione;istradefylline;KW-6002;10mg 50mg 100mg 1g 5g | | CAS: | 155270-99-8 | | MF: | C20H24N4O4 | | MW: | 384.43 | | EINECS: | | | Product Categories: | Inhibitors | | Mol File: | 155270-99-8.mol | ![8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione Structure](CAS/GIF/155270-99-8.gif) |
| | 8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione Chemical Properties |
| Melting point | 191 °C | | Boiling point | 601.0±65.0 °C(Predicted) | | density | 1.24±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | DMSO: soluble5mg/mL (clear solution) | | pka | 0.47±0.70(Predicted) | | form | powder | | color | white to beige | | InChIKey | IQVRBWUUXZMOPW-PKNBQFBNSA-N | | CAS DataBase Reference | 155270-99-8 |
| Hazard Codes | T | | Risk Statements | 25 | | Safety Statements | 45 | | RIDADR | UN 2811 6.1 / PGIII | | WGK Germany | 3 | | HS Code | 2933.99.7500 |
| | 8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione Usage And Synthesis |
| Description | In March 2013, istradefylline (also known as KW-6002) was approved in Japan for adjunctive treatment of Parkinson’s disease (PD). Istradefylline acts by antagonism of the adenosine A2A receptor, which is colocalized with dopamine D2 receptors in the striatum, to enhance dopamine D2-dependent signaling. Istradefylline is a light-sensitive compound and has been evaluated in vitro under low-light conditions to prevent isomerization of the (E)-styryl group and decomposition. Istradefylline has a Ki of 2.2 nM for the rat adenosine A2A receptor and an ED50 of 0.03 mg/kg, po, in reversal of haloperidol-induced catalepsy in mice. Further characterization showed istradefylline to have a Ki of 12 nM for the human adenosine A2A receptor, to be highly selective, and to be a functional competitive antagonist. Istradefylline has activity alone and in combination with levo-dopa in preclinical animal models of PD. The synthesis of istradefylline was accomplished by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide-mediated coupling of 5,6-diamino-1,3-diethyluracil with 3,4-dimethoxycinnamic acid, followed by cyclization upon treatment with aqueous NaOH, and selective methylation (MeI, K2CO3, and DMF). | | Description | Istradefylline is an adenosine receptor 2A (A2A) antagonist (Ki = 2.2 nM in a radioligand binding assay). In vivo, istradefylline inhibits catalepsy induced by haloperidol with an ED50 value of 0.23 mg/kg in rats. Oral administration of istradefylline alleviates postural defects in a dose-dependent manner without inducing dyskinesias or hyperactivity in an MPTP-induced marmoset model of Parkinson''s disease. It also decreases bradykinesias induced by L-DOPA and improves attentional and working memory deficits in an MPTP-induced macaque model of Parkinson''s disease. Formulations containing istradefylline are used to extend on-time in Parkinson''s disease patients experiencing motor fluctuations. | | Originator | Kyowa Hakko Kirin (Japan) | | Uses | Treatment of
Parkinson’s disease (adenosine A 2A receptor antagonist). | | Definition | ChEBI: Istradefylline is an oxopurine. | | Brand name | Nouriast | | Biochem/physiol Actions | Istradefylline (KW-6002) is a potent and selective adenosine A2A receptor selective antagonist which has been investigated for use in Parkinson′s Disease. | | Synthesis | Numerous synthetic approaches to istradefylline have been
developed, with a large majority of these methods employing
5,6-amino-1,3-diethyluracil 97 as a key intermediate.
Despite the commercial availability of 96, most reported routes
to istradefylline rely on sourcing of this intermediate via a wellestablished
four-step synthesis from N,N-diethylurea (94) and cyanoacetic
acid (95). Specifically, 6-amino-1,3-diethyluracil
(96) can be formed by sequential treatment of 94 and 95 with Ac2O
and NaOH. Nitrosation of 96 with NaNO2/AcOH/H2O, followed by
Na2S2O4/NH3-mediated nitroso reduction provided 5,6-amino-
1,3-diethyluracil (97).
Even though other groups have recently reported modified scale
routes to istradefylline, the route described herein will focus on
the sequence outlined by Kyowa Hakko Kogyo research laboratories
during their initial development of istradefylline.
EDC-mediated amine coupling involving 97 and 3,4-dimethoxycinnamic
acid (98) led to the corresponding amide intermediate. After
aqueous workup, this crude amide intermediate underwent cyclization with aqueous sodium hydroxide to yield the desired
purine dione 99 in 47% yield over 2 steps. Methylation of 99 with
MeI/K2CO3 provided istradefylline (XII) in 68% yield.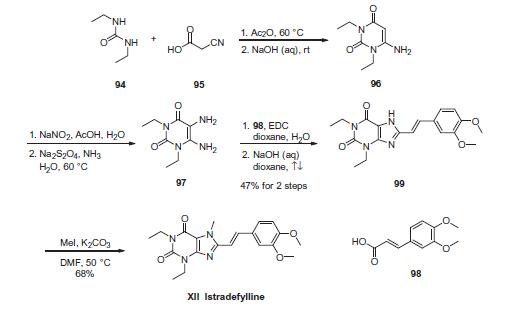
| | in vitro | the affinity of kw-6002 for the a2ar is 70-fold greater than that for the a1 receptor. the binding affinities (ki) of kw-6002 for human a1 receptor and a2a receptor are >287 nm and 9.12 nm, respectively [1]. | | in vivo | in mptp neurotoxin model of pd in mice, kw-6002 significantly attenuated striatal dopamine depletion under various conditions. in addition, pretreatment with kw-6002 (3.3 mg/kg, i.p.) before a single dose of mptp attenuated the partial dopamine and dopac depletions 1 week later [2]. | | storage | +4°C | | references | [1] park a, stacy m. istradefylline for the treatment of parkinson's disease. expert opin pharmacother. 2012 jan;13(1):111-4.
[2] chen jf, xu k, petzer jp, staal r, xu yh, beilstein m, sonsalla pk, castagnoli k, castagnoli n jr, schwarzschild ma. neuroprotection by caffeine and a(2a) adenosine receptor inactivation in a model of parkinson's disease. j neurosci. 2001;21(10):rc143. |
| | 8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione Preparation Products And Raw materials |
|