|
| | Lapatinib Ditosylate Basic information |
| | Lapatinib Ditosylate Chemical Properties |
| Melting point | 240-2420C | | storage temp. | Sealed in dry,Store in freezer, under -20°C | | solubility | ≥24.3 mg/mL in DMSO; insoluble in EtOH; insoluble in H2O | | form | solid | | Water Solubility | Water: Insoluble | | CAS DataBase Reference | 388082-77-7 |
| Safety Statements | 24/25 | | HS Code | 29420000 |
| | Lapatinib Ditosylate Usage And Synthesis |
| Description | Lapatinib, an ErB-1 and ErB-2 dual kinase inhibitor, was
launched for the treatment of advanced or metastatic HER2
(ErbB2) positive breast cancer in women who have received prior therapy. The drug was discovered and developed
by GlaxoSmithKline and is also currently being evaluated
for several additional cancer indications. | | Chemical Properties | Yellow Solid | | Uses | Lapatinib Ditosylate (GW572016, GW2016, Tykerb, Tyverb) is a potent EGFR and ErbB2 inhibitor with IC50 of 10.8 and 9.2 nM, respectively. | | Uses | Lapatinib Ditosylate is a reversible dual inhibitor of ErbB1 and ErbB2 tyrosine kinases. Antineoplastic. | | Synthesis | The synthesis started
with Williamson ether synthesis between 2-chloro-4-nitrophenol
(58) and 3-fluorobenzyl bromide to give ether 59 in the following scheme; however, no specific yields were provided.
Reduction of the nitro group of compound 59 by catalytic
hydrogenation over Pt/C and subsequent condensation of the
resulting aniline with 4-chloro-6-iodoquinazoline (61) in
refluxing i-PrOH afforded compound 62. 4-Chloro-6-iodoquinazoline
(61) was prepared by reacting 6-iodoquinazolin-
4(3H)-one (60) with POCl3 in the presence of triethylamine.
Compound 62 was subjected to Stille coupling with 5-
dioxolanyl-2-(tributylstannyl)furan (63) in the presence of
PdCl2(PPh3)2 to give 64. Acidic hydrolysis of acetal 64 using
HCl in THF/H2O provided the corresponding aldehyde which
was further subjected to reductive amination with 2-(methan-esulfonyl)ethylamine in the presence of sodium triacetoxyborohydride
to yield lapatinib. Lapatinib was treated with ptoluenesulfonic
acid solution to give lapatinib ditosylate
(IX). 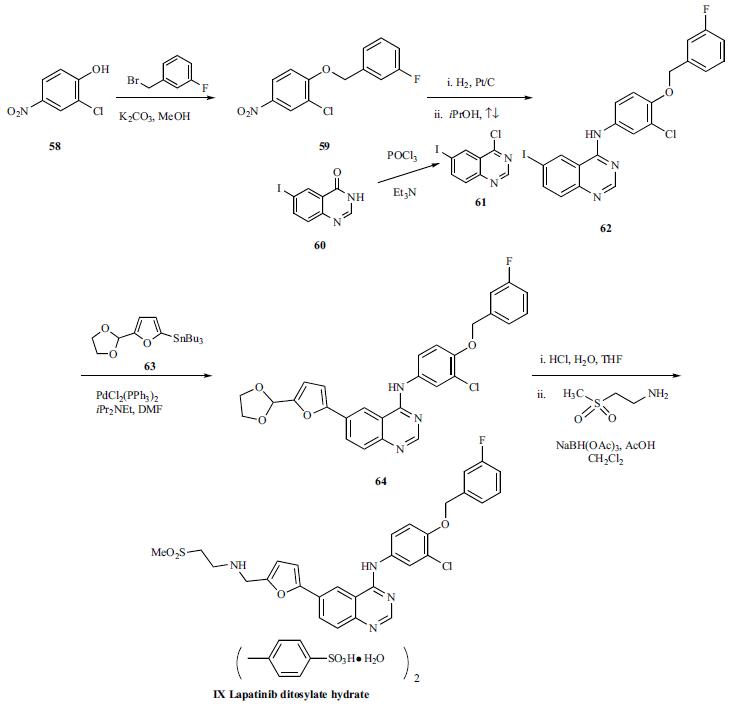
|
| | Lapatinib Ditosylate Preparation Products And Raw materials |
|