|
| | N,O-BIS(TRIMETHYLSILYL)HYDROXYLAMINE Basic information |
| Product Name: | N,O-BIS(TRIMETHYLSILYL)HYDROXYLAMINE | | Synonyms: | 2,2,5,5-Tetramethyl-3-oxa-4-aza-2,5-disilahexane;N,O-Bis-(trimethylsilyl)-hydroxylamin;Silanamine, 1,1,1-trimethyl-N-[(trimethylsilyl)oxy]-;1,1,1-trimethyl-N-[(trimethylsilyl)oxy]silylamine;N,O-BIS(TRIMETHYLSILYL)HYDROXYLAMINE, 97 %;N,0-Bis(trimethylsilyl)hydroxylamine;Bis(trimethylsilyl)hydroxylamine;(Trimethylsiloxy)(trimethylsilyl)amine | | CAS: | 22737-37-7 | | MF: | C6H19NOSi2 | | MW: | 177.39 | | EINECS: | 245-188-7 | | Product Categories: | Building Blocks;Chemical Synthesis;Nitrogen Compounds;Organic Building Blocks;Hydroxylamines;Hydroxylamines (N-Substituted);Hydroxylamines (O-Substituted);Si (Classes of Silicon Compounds);Si-N Compounds;Trimethylsilylazide, etc.;Nitrogen Compounds;Organic Building Blocks | | Mol File: | 22737-37-7.mol |  |
| | N,O-BIS(TRIMETHYLSILYL)HYDROXYLAMINE Chemical Properties |
| Boiling point | 78-80 °C100 mm Hg(lit.) | | density | 0.83 g/mL at 25 °C(lit.) | | refractive index | n20/D 1.411(lit.) | | Fp | 84 °F | | storage temp. | 2-8°C | | solubility | sol THF, ether, pentane, CH2Cl2. | | pka | 5.94±0.70(Predicted) | | form | clear liquid | | color | Colorless to Almost colorless | | Specific Gravity | 0.83 | | Hydrolytic Sensitivity | 7: reacts slowly with moisture/water | | BRN | 1920452 | | CAS DataBase Reference | 22737-37-7(CAS DataBase Reference) |
| | N,O-BIS(TRIMETHYLSILYL)HYDROXYLAMINE Usage And Synthesis |
| Chemical Properties | Clear colorless liquid | | Physical properties | bp 137–139°C, 78–80°C/100 mmHg; d 0.830
g cm?3; flash point 28°C. | | Uses | N,O-Bis(trimethylsilyl)hydroxylamine has been used as reagent for the preparation of N-(dialkyl phosphinoyl)hydroxylamines and migration of simple alkyl groups in rearrangement of their O-p-nitrobenzenesulfonates. | | Uses | N,O-Bis(trimethylsilyl)hydroxylamine
is a protected, lipophilic form of hydroxylamine. It reacts
with a variety of electrophiles predominantly by attack on the nitrogen
nucleophilic center. Reaction with acid chlorides (1 equiv)
in the presence of triethylamine gives N,O-bis(trimethylsilyl)-
hydroxamic acids by N-acylation.A related reagent, tris(trimethylsilyl)
hydroxylamine, gives the same product in high yields,
also by N-acylation.Hydrolysis gives the free hydroxamic
acids, whereas thermal fragmentation affords isocyanates (eq 1).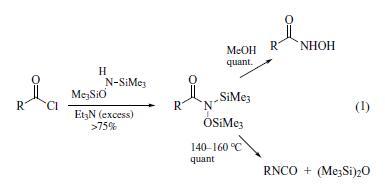 | | Preparation | N,O-Bis(trimethylsilyl)hydroxylamine can be prepared in 69% yield by treating
dry hydroxylamine with equiv chlorotrimethylsilane and
equiv triethylamine.A safer preparation, which avoids the
use of the explosive solid, hydroxylamine, uses the reaction
of hydroxylamine hydrochloride with excess hexamethyldisilazane
(71–75% yield).Alternatively, neutralization of hydroxylamine
hydrochloride with ethylenediamine followed by
addition of chlorotrimethylsilane can be used. | | General Description | N,O-Bis(trimethylsilyl)hydroxylamine on reaction with 9-chloro-9-borafluorene yields 10-trimethylsilyloxy-9-aza-10-boraphenanthrene. It reacts with free phenolic steroids during the analysis of conjugated estrogen tablets and injectable formulations by GLC. |
| | N,O-BIS(TRIMETHYLSILYL)HYDROXYLAMINE Preparation Products And Raw materials |
|