|
| | Hippuric acid Chemical Properties |
| Melting point | 187-191 °C (lit.) | | Boiling point | 311.69°C (rough estimate) | | density | 1,371 g/cm3 | | refractive index | 1.5600 (estimate) | | storage temp. | Store below +30°C. | | solubility | 3.26g/l | | pka | 3.62(at 25℃) | | form | Crystalline Powder | | color | White to almost white | | PH | 2.5-3.5 (10g/l, H2O, 20℃)(slurry) | | Water Solubility | Soluble in water. | | Merck | 14,4716 | | BRN | 1073987 | | Stability: | Stable. Incompatible with oxidizing agents. | | InChIKey | QIAFMBKCNZACKA-UHFFFAOYSA-N | | LogP | 0.771 | | CAS DataBase Reference | 495-69-2(CAS DataBase Reference) | | EPA Substance Registry System | Glycine, N-benzoyl- (495-69-2) |
| | Hippuric acid Usage And Synthesis |
| Physical and Chemical Properties | Hippuric acid is also known as "Benzoylaminoethanoic acid",its molecular formula is C9H9NO3. The molecular weight is 179.18. As colorless crystals. Melting point 187~188 ℃. It's soluble in cold water, chloroform, ether, cold ethanol, slightly soluble in amyl alcohol, soluble in hot water, hot ethanol, sodium phosphate solution, almost insoluble in benzene, carbon disulfide, petroleum ether. Naturally it occurs in herbivores (such as horses) of urine, it is also present in small amounts in human urine,it is easily hydrolyzed to benzoic acid and glycine. Hippuric acid can reduce urine pH within a certain range,which makes the bacteria living environment change, and then plays a synergistic bactericidal effect. Urinary hippuric acid is measured using pyridine-benzene chloride colorimetry. The principle is that hippuric acid reacts with benzene sulfonyl chloride in pyridine solution, and the product in ethanol solution is yellow compound, according to the color depth, quantitative analysis is processed by colorimetry. Normal urine hippuric acid content varies widely because of different varieties of diet and intake (from 0.3 to 2.5 grams per day), and there are individual differences. After Toluene goes into the body, through metabolic transformation, most of it becomes to hippuric acid and is excreted through urine. Determination of horse urine hippuric acid content can be used as exposure indicators to reflect the body's absorption of toluene, but it can not serve as indicators of the diagnosis.
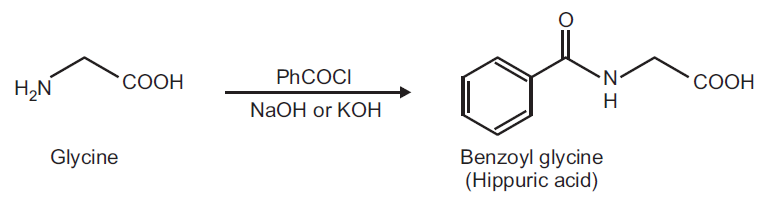
Hippuric acid can be made by reaction of benzoyl chloride with glycine in sodium hydroxide solution . It can be used for biochemical reagents and organic synthesis. Hippuric acid ammonium salt is crystal, soluble in water, soluble in ethanol; potassium salt is crystal, containing single molecule of crystal water, soluble in water, soluble in ethanol.
Properties, uses, methods of synthesis of hippuric acid Information compiled by Andy of ChemicalBook (2016-11-19). | | Hippuric acid excretion test | The use of the principles of sodium benzoate going into the human body and binding glycine in the liver to be non-toxic hippuric acid and being excreted with the urine,can be used to exam liver detoxification function, which is called hippuric acid excretion test. The kidneys can not only excrete hippuric acid, it can also synthesize a small amount of hippuric acid .It is only in normal renal function that hippuric acid test can truly reflect the liver's detoxification function using hippuric acid excretion test . A little after the subjects take breakfast, oral 6g sodium benzoate is taken. 4h after taking the drug, collect the urine, measure the urine excretion, the normal amount ofhippuric acid after 4h discharge should be> 3.0g. To avoid nausea and vomiting induced often by oral sodium benzoate , intravenous injection 1.77g sodium benzoate can be slowly done ,urine is collected 1h after injection, discharge amount is measured , the normal discharge amount should be 1h> 0.7g. When there is liver parenchymal disease, such as hepatitis, cirrhosis and liver necrosis, the liver synthesis of hippuric acid dysfunctions, which decreases production of hippuric acid . Obstructive jaundice causes small changes. Reduced renal blood flow and renal excretion dysfunction also lead to the reducing of the amount of hippuric acid discharge . Some fruits contain benzoic acid such as apples, bananas, etc., they should be banned when testing. Hippuric acid test has many factors and sodium benzoate can cause adverse reactions in patients, and the valuation of liver detoxification function is less precise, it has been less clinical.
Reference: China Medical Encyclopedia twenty-seven DIAGNOSIS [author:Sun Rongwu] | | Uses | The product is used for medicine, dyes intermediates and it is used for the production of fluorescent yellow H8GL, disperse fluorescent FFL and so on. | | production method | Benzoyl chloride reacts with the amino acid in sodium hydroxide solution, amido sodium benzene is obtained, then it is acidized with hydrochloric acid. The amino acid is dissolved in sodium hydroxide solution, at the same time drop benzoyl chloride and sodium hydroxide solution below 30 ℃ , the reaction solution is always alkaline. After finishing adding, stir for 30min. Then add hydrochloric acid to pH 2, filter crude, use water to recrystallize , hippuric acid is obtained. Consumption of raw materials fixed: amino acid (90%) 610kg/t, benzoyl chloride (60%) 1520kg/t, caustic soda (30%) 1960kg/t, hydrochloric acid (30%) 900kg/t. | | Chemical Properties | White crystalline powder | | Uses | N-Benzoylglycine also known as Hippuric Acid is the glycine conjugate of benzoic acid commonly found in ruminant urine. It is synthesized in the liver and its production is greatly increased following consuption of benzoic acid. In itself it does not have a direct biological function, however p-hydroxy-hippurica acid can be used as an inhibitor of Ca2+ ATPase. | | Uses | Hippuric acid is used as a intermediate for the manufacturing medicine and other organic compounds. Hippuric acid can be used to study cell biology, chemical biology, bioactive small molecules, amino acid derivatives, peptide synthesis, chemical synthesis and nutrition. Hippuric acid has been used to inform the metabolism and urinary excretion of procyanidins. | | Preparation | Preparation of Hippuric acid from Glycine.
Principle: The hydroxy and amino functions can be easily benzoylated using Benzoyl chloride in aqueous alkaline conditions. This reaction is known as Schotten-Baumann reaction.
Reaction:
Procedure: Dissolve 0.5 g of glycine in 5 ml of 10% NaOH solution in a 25 ml conical flask. Add 0.9 ml of benzoyl chloride in five portions to the solution. Stopper the flask and shake vigorously after each addition until all the benzoyl chloride is reacted. Transfer the solution to a beaker and add few grams of ice and add conc. HCl slowly with stirring until the solution is acidic to litmus paper. Filter the crystalline precipitate of the product on a Buchner funnel, wash with cold water and drain well. Place the solid in a conical flask with 10 ml CCl4 and boil gently for 10 min. Allow the mixture to cool slightly, filter under gentle suction and wash with 10-20 ml CCl4. Record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product from boiling water (5 ml), with addition of decolorizing charcoal if necessary, filter the hot solution and cool the filtrate. The crystals of the product separate out. Filter, dry and record the melting point and TLC [using Butanal : acetic acid: water (4 : 1 : 1) as solvent or CHCl3:methanol (9 : 1) or toluene].
| | Definition | ChEBI: An N-acylglycine in which the acyl group is specified as benzoyl. | | Purification Methods | Crystallise the acid from boiling H2O. Dry it over P2O5. Also purify it by dissolving 135-140g in 2L of boiling H2O, filtering through a steam-heated funnel and allowing to crystallise at ~20o (yield 115-122g first crop, m 186-187o). [Ingersoll & Babcock Org Synth Coll Vol II 328 1943, Beilstein 9 225, I 100.] |
| | Hippuric acid Preparation Products And Raw materials |
|



