|
| | Norethindrone Basic information |
| | Norethindrone Chemical Properties |
| Melting point | 205-206 °C (lit.) | | alpha | D20 -31.7° (chloroform); D20 -25° (chloroform) | | Boiling point | 379.83°C (rough estimate) | | density | 1.0766 (rough estimate) | | refractive index | 1.4800 (estimate) | | storage temp. | 2-8°C | | solubility | chloroform: ≥50 mg/mL, clear, colorless | | pka | 13.09±0.40(Predicted) | | form | powder | | color | white to off-white | | Water Solubility | 7.043mg/L(25 ºC) | | Merck | 6697 | | BRN | 1915671 | | BCS Class | 1 | | InChIKey | VIKNJXKGJWUCNN-XGXHKTLJSA-N | | CAS DataBase Reference | 68-22-4(CAS DataBase Reference) | | NIST Chemistry Reference | Norethindrone(68-22-4) | | EPA Substance Registry System | Norethisterone (68-22-4) |
| | Norethindrone Usage And Synthesis |
| Description | Norethindrone is a progestin (a synthetic substance with properties similar to progesterone)
that is best known as the first female oral contraceptive, or the “pill.”Norethindrone’s global
impact on society and culture has made it one of the most important inventions in history. | | Chemical Properties | Off-White to Pale Yellow Solid | | Originator | Norlutin,Parke Davis ,US,1957 | | History | The development of norethindrone as a female oral contraceptive took place indirectly over 30 years as a result of steroid research.This research accelerated in the 1930s when structures and medical applications of steroidal compounds were determined.Steroids are lipids, which include cholesterol, bile salts,and sex hormones,that are characterized by a structure of three fused six-carbon rings and a five-carbon ring.
In 1957, both norethindrone and norethynodrel were approved by the Food and Drug Administration (FDA) for treating menstrual problems and infertility. In 1960, the FDA approved Searle's norethynodrel under the trade name Enovid. Norethindrone was approved as an oral contraceptive in 1962 under the trade name Ortho-Novum. | | Uses | Progestin contraceptives work by producing pregnant-like conditions in a female to preventovulation.During pregnancy, progesterone is released by the placenta during development ofthe fetus.This in turn suppresses development of egg follicles and ovulation. Progestins mimicthis condition and thus prevent or delay ovulation.Oral contraceptives currently use progestinand estrogen in combination to prevent ovulation and thicken cervical mucus.The latter makeit harder for sperm to enter the uterus and for an egg to implant on the uterine wall. | | Uses | progestogen | | Uses | A synthetic progestin | | Uses | Progesteron. Norethindrone and acetate in combination with estrogen as contraceptive (oral). It is reasonably anticipated to be a human carcinogen | | Definition | ChEBI: A 17beta-hydroxy steroid that is testosterone in which the hydrogen at position 17 is replaced by an ethynyl group and in which the methyl group attached to position 10 is replaced by hydrogen. | | Manufacturing Process | 7.5 grams of 3-methoxyestrone were dissolved in 750 cc of anhydrous dioxane in a three-neck flask, placed in a box and insulated with cotton wool. 2 liters of anhydrous liquid ammonia and 15 grams of lithium metal in the form of wire were added to the mechanically stirred solution. After stirring for one hour, 150 cc of absolute ethanol were added at such speed that no bumping occurred; when the blue color had disappeared, 500 cc of water were added in the same way. The ammonia was evaporated on the steam bath and the product collected with 2 liters of water. It was extracted with ether and then with ethyl acetate and the combined extract was washed to neutral and evaporated to dryness under vacuum, leaving 7.4 grams of a slightly yellow oil.
The oil thus obtained was dissolved in 400 cc of methanol and refluxed during one hour with 150 cc of 4N hydrochloric acid. The mixture was poured into a sodium chloride solution and extracted with ethyl acetate, washed to neutral, dried and evaporated to dryness. The product was a yellow oil which showed an ultraviolet absorption maximum characteristic of a ?4-3-ketone.
A solution of 2.7 grams of chromic acid in 20 cc of water and 50 cc of acetic acid was added to the stirred solution of the above oil in 100 cc of acetic acid, maintaining the temperature below 20°C. After 90 minutes standing, 50 cc of methanol were added and the mixture concentrated under vacuum (20 mm). The residue was extracted with ether, washed to neutral and evaporated to dryness. The residual semicrystalline product (7 grams) was chromatographed over alumina and the fractions eluted with ether yielded 3.2 grams of ?4-19norandrosten-3,17-dione having a MP of 163° to 167°C.
A solution of 2 grams of ?4-19-norandrosten-3,17-dione and 0.4 gram of pyridine hydrochloride in 50 cc of benzene free of thiophene was made free of moisture by distilling a small portion; 4 cc of absolute alcohol and 4 cc of ethyl orthoformate were added and the mixture was refluxed during 3 hours. 5 cc of the mixture were then distilled and after adding an additional 4 cc of ethyl orthoformate the refluxing was continued for 2 hours longer. The mixture was evaporated to dryness under vacuum and the residue was taken up in ether, washed, dried and evaporated to dryness. The residue was crystallized from hexane-acetone and then from ether to give ?3,5-19-nor-3ethoxy-androstadien-17-onewith a MP of 140° to 142°C.
One gram of potassium metal was dissolved in 25 cc of tertiary amyl alcohol by heating under an atmosphere of nitrogen. One gram of ?3,5-19-nor-3ethoxyandrostadien-17-onein 25 cc of anhydrous toluene was added and nitrogen was passed during 15 minutes. Then acetylene (especially dried and purified) was passed during 14 hours through the mechanically stirred solution, at room temperature.
The mixture was poured in water, acidified to pH 1 with dilute hydrochloric acid, heated on the steam bath for 30 minutes and then subjected to steam distillation to remove the organic solvents. The residue was filtered, dried and recystallized several times from ethyl acetate. The ?4-19-nor-17αethinylandrosten-17β-ol-3-onethus obtained had a MP of 198° to 200°C (in sulfuric acid bath), 200° to 204°C (Kofler).
| | Brand name | Camila (Barr); Errin (Barr); Micronor (OrthoMcNeil); Nor-QD (Watson); Norlutin (Parke-Davis). | | Therapeutic Function | Progestin | | General Description | Norethindrone, 17α-ethinyl-19-nortestosterone, and itsΔ5(10)-isomer, norethynodrel, might appear at first glance tobe subtle copies of each other. One would predict that theΔ5(10)-double bond would isomerize in the stomach’s acid tothe Δ4-position. However, the two drugs were actually developedsimultaneously and independently; hence, neither can beconsidered a copy of the other. Furthermore, norethindrone isabout 10 times more active than norethynodrel, indicating that isomerization is not as facile in vivo asone might predict. Although they are less active than progesteronewhen given subcutaneously, they have the importantadvantage of being orally active. The discovery of the potentprogestin activity of 17α-ethinyltestosterone (ethisterone) and19-norprogesterone preceded the development of these potentprogestins. Both are orally active, with the 17α-ethinyl groupblocking oxidation to the less active 17-one. The rich electrondensity of the ethinyl group and the absence of the 19-methylgroup greatly enhance progestin activity. Both compoundswere of great importance as progestin components of oralcontraceptives, although currently, use of norethynodrel isminimal. Norethindrone, USP, and norethindrone acetate,USP, are widely used for all the usual indications of the progestins,as well as being components of oral contraceptives.Because these compounds retain key features of the testosteronestructure, including the 17β-OH, it is not surprisingthat they possess some androgenic side effects. | | Biochem/physiol Actions | 19-norethindrone is an oral contraceptive involved in the inhibition of cytosolic sulfotransferases (SULT). | | Clinical Use | Progestogen:
Breast cancer, contraception, dysfunctional
uterine bleeding, menorrhagia, dysmenorrhoea,
endometriosis, premenstrual syndrome,
postponement of menstruation | | Safety Profile | Confirmed carcinogen
with experimental carcinogenic,
tumorigenic, and teratogenic data. Mddly
toxic by ingestion. Human systemic effects
by ingestion: dermatitis and androgenic
effects. Human teratogenic effects:
developmental abnormalities of the
musculoskeletal system and urogenital
system; and behavioral effects in the
newborn. Human reproductive effects:
spermatogenesis; testes, epididymis, sperm
duct changes; impotence; male breast
development; other male effects; ovaries,
fallopian tube changes; menstrual cycle effects; postpartum effects; changes in
female fertility. Experimental reproductive
effects. Human mutation data reported.
When heated to decomposition it emits
acrid smoke and irritating fumes. | | Synthesis | Norethindrone, 17|á-ethynyl-17|?-hydroxyestra-4-en-4-one (28.3.12),
is made from 19-nor-4-androsten-3,17-dione (28.3.10), which is in turn synthesized by
partial reduction of the aromatic region of the 3-O-methyl ether of estrone with lithium
in liquid ammonia, and simultaneously of the keto-group at C17 to and hydroxyl group,
which is then oxidized back to a keto-group by chromium (VI) oxide in acetic acid. The
conjugated with the double bond carbonyl group at C3 is then transformed to dienol ethyl
ether (28.3.11) using ethyl orthoformate. The obtained product is ethynylated by acetylene
in the presence of potassium tert-butoxide. After hydrochloric acid hydrolysis, of
the formed O-potassium derivative, during which the enol ether is also hydrolyzed, and
the remaining double bond is shifted, the desired norethindrone (28.3.12) is obtained. 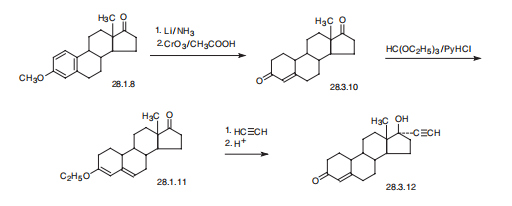
| | Drug interactions | Potentially hazardous interactions with other drugs
Antibacterials: metabolism of progestogens
accelerated by rifamycins (reduced contraceptive
effect).
Anticoagulants: progestogens antagonise
anticoagulant effect of phenindione; may enhance or
reduce anticoagulant effect of coumarins.
Antidepressants: contraceptive effect reduced by St
John’s Wort - avoid.
Antiepileptics: metabolism accelerated by
carbamazepine, eslicarbazepine, fosphenytoin,
lamotrigine, oxcarbazepine, phenobarbital,
phenytoin, rufinamide and topiramate (reduced
contraceptive effect); concentration of lamotrigine
reduced; concentration reduced by high dose
perampanel.
Antifungals: reduced contraceptive effect with
griseofulvin.
Antivirals: contraceptive effect reduced by
efavirenz; metabolism accelerated by nevirapine
(reduced contraceptive effect); atazanavir increases
norethisterone concentration.
Aprepitant: possible contraceptive failure.
Bosentan: possible contraceptive failure.
Ciclosporin: progestogens inhibit metabolism of
ciclosporin (increased plasma concentration).
Cytotoxics: possibly reduced contraceptive effect with
crizotinib, dabrafenib, olaparib and vemurafenib.
Dopaminergics: concentration of selegiline increased
- avoid.
Fosaprepitant: possible contraceptive failure.
Lumacaftor: possible contraceptive failure.
Tacrolimus: tacrolimus levels are greatly increased -
avoid (anecdotal evidence).
Ulipristal: contraceptive effect of progestogens
possibly reduced. | | Carcinogenicity | Norethisterone is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. | | Environmental Fate | Waste streams from manufacturing plants producing contraceptives
containing norethisterone can be sources of its release
to the environment. If released to the air, norethisterone exists in
both vapor and particulate phases in the atmosphere as deduced
from a vapor pressure of 3.1× 10-7mmHg at 20 ℃. This vapor
pressure indicates that norethisterone is not expected to be
volatile from dry soil surfaces. Furthermore, based on an estimated
Henry’s law constant of 5.8×10-10 atm m3 mol-1 for
norethisterone, volatilization from water and moist soil surfaces
is not plausible.
In aquatic systems, norethisterone is expected to adsorb to
suspended solids and sediments given by its Koc value (soil
organic carbon–water partitioning coefficient) of 220.
In the air, the vapor phase of norethisterone can be
degraded by reaction with photochemically produced hydroxyl
radicals with an estimated half-life of 1.1 h; the particulate
phase can be removed by wet or dry deposition. Norethisterone
is likely susceptible to photolysis by sunlight because of the
presence of chromophores that absorb at wavelengths more
than 290 nm. Hydrolysis of norethisterone is not anticipated
under environmental conditions because of the lack of a functional
group to hydrolyze.
In terrestrial systems, the Koc value of 220 suggests that
norethisterone has moderate mobility in soil.
An estimated bioconcentration factor (BFC) of 42 for norethisterone
indicates that its potential for bioconcentration in
aquatic organisms is moderate. | | Metabolism | It is metabolised in the liver with 50-80% of a dose being
excreted in the urine and up to 40% appearing in the faeces. | | Toxicity evaluation | As a synthetic progestin, norethisterone enters the target cells
by passive diffusion and binds to its intracellular receptor to
initiate transcription and protein synthesis. It changes the
cervical mucus so that sperm migration or implantation of
the fertilized ovum in the uterus is inhibited. Repeated low
doses of norethisterone can change the rate of ovum transport
by affecting motility and secretion in the fallopian
tubes. When administered at high doses, norethisterone can
suppress ovulation and cause ovarian and endometrial
atrophy. Variable suppression of follicle stimulating
hormone (FSH) and luteinizing hormone (LH) occurs with
low doses. |
| | Norethindrone Preparation Products And Raw materials |
| Raw materials | Tetrahydrofuran-->Ammonia-->Lithium-->Potassium-->Acetylene-->Chromic acid-->Triethyl orthoformate-->Estrone 3-methyl ether-->Norandrostenedione-->19-ALDOANDROSTENEDIONE-->3-Ethoxyestra-3,5-dien-17-one-->3-methoxyestra-2,5(10)-dien-17beta-ol-->19-Norpregna-2,5(10)-dien-20-yn-17-ol, 3-methoxy-, (17α)- (9CI)-->19-HYDROXY-4-ANDROSTENE-3,17-DIONE-->19-CARBOXYANDROST-4-ENE-3,17-DIONE-->Norethindrone-3-ethyldienolether-->Nandrolone | | Preparation Products | 19-Nor-17-alpha-pregna-3,5-dien-20-yne-3,17-diol, diacetate |
|



