|
| | Nintedanib Ethanesulfonate Salt Basic information |
| Product Name: | Nintedanib Ethanesulfonate Salt | | Synonyms: | (3Z)-2,3-Dihydro-3-[[[4-[methyl[2-(4-methyl-1-piperazinyl)acetyl]amino]phenyl]amino]phenylmethylene]-2-oxo-1H-indole-6-carboxylic acid methyl ester ethanesulfonate;Nintedanib ethanesulfonate;BIBF 1120 esylate;BIBF-1120 esylate;Nintedanib esylate;BIBF1120/nintedanib ethanesulfonate salt;Trinidad Neeb esylate;Intedanib ethanesulfonate | | CAS: | 656247-18-6 | | MF: | C33H39N5O7S | | MW: | 649.76 | | EINECS: | | | Product Categories: | API | | Mol File: | 656247-18-6.mol |  |
| | Nintedanib Ethanesulfonate Salt Chemical Properties |
| Melting point | >233°C (dec.) | | storage temp. | -20°C Freezer | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Yellow solid. | | color | Light Yellow to Yellow |
| | Nintedanib Ethanesulfonate Salt Usage And Synthesis |
| Description | Nintedanib esylate is a potent, oral triple angiokinase inhibitor
developed by Boehringer Ingelheim that targets proangiogenic and
pro-fibrotic pathways mediated by the vascular endothelial growth
factor receptor, fibroblast growth factor receptor and plateletderived
growth factor receptor families, as well as Src and Flt-3
kinases. It was approved for the treatment of idiopathic pulmonary
fibrosis (IPF), a condition in which the lungs become progressively
scarred over time, by the US FDA in October 2014 and by
the EMA in January 2015. The FDA granted nintedanib esylate
fast-track, priority review, orphan product, and breakthrough
designations. The drug was also approved by the EMA in November
2014 for treatment of non-small cell lung cancer in combination
with docetaxel after first-line chemotherapy. | | Uses | Nintedanib Esylate is the salt form of Nintedanib, which is angiokinase inhibitor and is used in the treatment of idiopathic pulmonary fibrosis. Also inhibits the process blood vessel formation which may be used to assist in cancer therapy. | | Definition | ChEBI: An organosulfonate salt obtained by combining nintedanib with one molar equivalent of ethanesulfonic acid. A kinase inhibitor used for the treatment of idiopathic pulmonary fibrosis and cancer. | | Synthesis | The synthesis of indolinone 197 commenced with commercial
4-chloro-3-nitro-benzoic acid (194)?aesterification of which preceded
displacement of the chloride by dimethyl malonate (195)
in the presence of base to generate nitrobenzene 196. Hydrogenation
of 196 under acidic conditions furnished 6-methyoxycarbonyl-
substituted oxindole 197 by decarboxylative cyclization in
87% yield. Acylation of indolinone 197 with chloroacetic anhydride
in refluxing toluene and subsequent condensation with trimethyl
orthobenzoate resulted in indolone 198, which was isolated in
86% yield over the two-step sequence. While these two steps could
reportedly be combined into a one-pot protocol using acetic anhydride
as the solvent, the stepwise procedure was found to be more
amenable for large-scale synthesis due to fewer complications
with undesired side products. Subjection of 198 to methanolic
potassium hydroxide followed by condensation with aniline
fragment 199
in refluxing methanol and then exposure to aqueous ethanesulfonic
acid in methanol provided nintedanib esylate (XXIII) in 82%
over the three-step sequence.
Aniline fragment 199 was prepared in three steps
and 82% overall yield via initial acylation of N-methyl-4-nitroaniline
200 with chloro acetylchloride followed by displacement of
the a-amidochloride with N-methylpiperazine and hydrogenative
reduction of the nitro group gave the desired aniline.183,184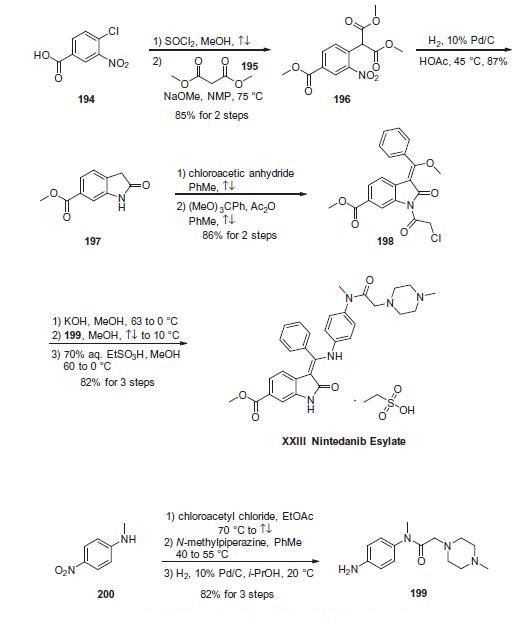
|
| | Nintedanib Ethanesulfonate Salt Preparation Products And Raw materials |
|



