|
| | Carisoprodol Basic information |
| | Carisoprodol Chemical Properties |
| Melting point | 92-92°C | | Boiling point | 403.59°C (rough estimate) | | density | 1.1035 (rough estimate) | | refractive index | 1.4560 (estimate) | | storage temp. | 2-8°C | | solubility | Very slightly soluble in water, freely soluble in acetone, in alcohol and in methylene chloride. | | form | Solid | | pka | pKa 4.2 (Uncertain) | | color | White to Off-White | | Water Solubility | <0.1 g/100 mL at 19.5 ºC | | BCS Class | 2/4 | | CAS DataBase Reference | 78-44-4(CAS DataBase Reference) | | NIST Chemistry Reference | Carisoprodol(78-44-4) | | EPA Substance Registry System | Carisoprodol (78-44-4) |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 36 | | RTECS | FB3325000 | | HS Code | 29224999 | | Hazardous Substances Data | 78-44-4(Hazardous Substances Data) | | Toxicity | LD50 in mice, rats (mg/kg): 2340, 1320 orally; 980, 450 i.p. (Berger) |
| | Carisoprodol Usage And Synthesis |
| Description | Carisoprodol (CRM) (Item No. ISO60206) is a certified reference material categorized as a skeletal muscle relaxant. It also has sedative properties. Carisoprodol is regulated as a Schedule IV compound in the United States. Carisoprodol (CRM) (Item No. ISO60206) is provided as a DEA exempt preparation. This product is intended for research and forensic applications. | | Description | Carisoprodol (Item No. 30778) is an analytical reference standard categorized as a skeletal muscle relaxant. It also has sedative properties. Carisoprodol is regulated as a Schedule IV compound in the United States. This product is intended for research and forensic applications. | | Chemical Properties | White Solid | | Originator | Soma,Wallace,US,1959 | | Uses | For the relief of discomfort associated with acute, painful, musculoskeletal conditions. | | Uses | Carisoprodol suppresses interneuronal action of reticular formation of the spinal cord. It is
used as an adjuvant drug for loss of flexibility of skeletal muscle as well as for relieving
pain caused therein. Synonyms of this drug are rela, soma, carisoma, and sanoma. | | Uses | Muscle relaxant (skeletal)
Carisoprodol has an onset of action of ca. 30min and a duration of 4–6h. It is administered orally. Presumably, it acts by inhibiting interneuronal activity in the spinal cord and the brainstem reticular formation. Clinically effective doses are accompanied by drowsiness or dizziness; its mechanism may involve sedation.Its CNS depressant effects are additive with those of ethanol and other psychotropic agents. Carisoprodol has a low potential for drug dependence.

| | Definition | ChEBI: A carbamate ester that is the mono-N-isopropyl derivative of meprobamate (which is a significant metabolite). Carisoprodol interrupts neuronal communication within the reticular formation and spinal cord, resulting in sedation and alter
tion in pain perception. It is used as a muscle relaxant in the symptomatic treatment of musculoskeletal conditions associated with painful muscle spasm. | | Preparation | Carisoprodol is Prepared by reaction of 2-methyl-2-propyl-1,3-pro-panediol with phosgene and ammonium hydroxide, then with isopropyl isocyanate. | | Manufacturing Process | A cooled 10% solution of 1 mol of phosgene in toluene was added with
stirring to a cooled solution of 1 mol of 2-methyl-2-propyl-1,3-propanediol and
2 mols of dimethylaniline also dissolved in toluene, at such a rate that the
temperature of the mixture was maintained at about 25°C. The mixture was
allowed to remain at this temperature for several hours, then cooled and
extracted with cold 5% hydrochloric acid solution to remove the
dimethylaniline. The toluene layer was dried using a suitable drying agent and
the 2-methyl-2-propyl-3-hydroxypropyl chlorocarbonate used in subsequent
reactions in the form of its solution in anhydrous toluene.
A quantity of solution obtained as described containing 0.1 mol of the
chlorocarbonate was treated with 0.2 mol of anhydrous isopropylamine and
allowed to react at ordinary room temperature. The solution was cooled,
extracted with dilute hydrochloric acid and the organic layer concentrated by
evaporation of the solvent. The crude monocarbamate was purified by
distilling at 86° to 88°C at about 0.01 mm. It was a clear, viscous liquid.
21.7 g (0.1 mol) of N-isopropyl-2-methyl-2-propyl-3-hydroxypropylcarbamate
and 7.5 g (0.11 mol) of anhydrous sodium cyanate are stirred in 200 ml
anhydrous chloroform in a suitable vessel equipped with a gas inlet tube,stirrer and thermometer. While cooling the vessel, anhydrous hydrogen
chloride is passed into the stirred mixture slowly for 5 hours maintaining the
temperature between 0° and 5°C. Alternatively ethyl urethane in the presence
of aluminum isopropylate as a catalyst may be used in place of the sodium
cyanates and HCl. The mixture is then allowed to stand at room temperature
overnight.
The solid material is separated by filtration and the chloroform solution
concentrated to an oil under reduced pressure. The oil is dissolved in 50 ml of
trichloroethylene, the solution treated with charcoal, filtered and the filtrate
added to 125 ml of hexane. The crystalline material which forms on standing
at refrigerator temperature is removed by filtration, washed with light
petroleum ether and dried at about 50°C. Approximately 20 g of product are
obtained. On recrystallizing from trichloroethylene-hexane, 17.8 g of purified
compound are obtained, MP 89° to 91°C. | | Brand name | Rela (Schering); Soma (Medpointe). | | Therapeutic Function | Muscle relaxant | | General Description | Carisoprodol, N-isopropyl-2-methyl-2-propyl-1,3-propanediol dicarbamate, 2-methyl-2-propyl-1,3-propanediol carbamate isopropylcarbamate(Soma), is the mono-N-isopropyl–substituted relative ofmeprobamate. The structure is given in the discussion ofmeprobamate. It is indicated in acute skeletomuscular conditionscharacterized by pain, stiffness, and spasm. As canbe expected, a major side effect of the drug is drowsiness. | | Air & Water Reactions | Insoluble in water. | | Reactivity Profile | Carisoprodol is a carbamate ester. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates. | | Health Hazard | SYMPTOMS: The most common symptoms of exposure to Carisoprodol are drowsiness and hives. Other symptoms may include nausea, vomiting, epigastric distress, vertigo, ataxia, tremors, agitation, irritability, headache, insomnia, fainting, hiccups, visual disturbances, asthma, fever, hypotension, excitement and paralysis. | | Fire Hazard | Flash point data for Carisoprodol are not available, but Carisoprodol it probably combustible. | | Synthesis | Carisoprodol, N-iso-propyl-2-methyl-2-propyl-1,3-propanediol (15.3.12),
is synthesized by reacting 2-methyl-2-propylpropanediol-1,3 dicarbamate with 1 mol of
phosgene, forming the chloroformate (15.3.10), from which carbamate (15.3.11) is formed
by reacting it with isopropylamine. Reacting this with either urethane or sodium cyanate
gives carisoprodol (15.3.12). 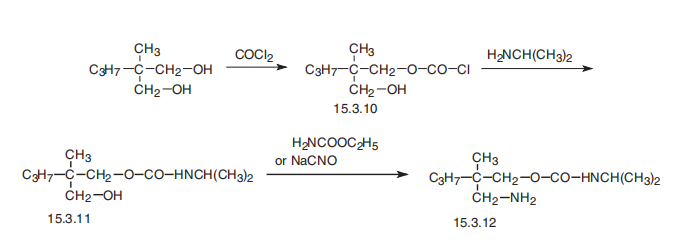
|
| | Carisoprodol Preparation Products And Raw materials |
|




