|
| | 1-CHLORO-6,6-DIMETHYL-2-HEPTEN-4-YNE Basic information |
| Product Name: | 1-CHLORO-6,6-DIMETHYL-2-HEPTEN-4-YNE | | Synonyms: | 2-HEPTEN-4-YNE, 1-CHLORO-6,6-DIMETHYL-, (2E)-;1-CHLORO-6,6-DIMETHYL-2-ENE-4-YNE-HEPTANE;1-Chloro-6,6-dimethyl-2-hepten-4-ino;2-HEPTEN-4-YNE,1-CHLORO-6,6-DIMETHYL;1-Chloro-6,6-dimethyl-2-hepten-4-yne;(E)-1-chloro-6,6-diMethylhept-2-en-4-yne;(2E)-1-chloro-6,6-dimethylhept-2-en-4-yne;Terbinafine Impurity 22 | | CAS: | 287471-30-1 | | MF: | C9H13Cl | | MW: | 156.65 | | EINECS: | 608-241-9 | | Product Categories: | Chemical intermediate for Terbinafine | | Mol File: | 287471-30-1.mol |  |
| | 1-CHLORO-6,6-DIMETHYL-2-HEPTEN-4-YNE Chemical Properties |
| Boiling point | 207.5±23.0 °C(Predicted) | | density | 0.946±0.06 g/cm3(Predicted) | | InChI | InChI=1S/C9H13Cl/c1-9(2,3)7-5-4-6-8-10/h4,6H,8H2,1-3H3/b6-4+ | | InChIKey | ZIXABMZBMHDFEZ-GQCTYLIASA-N | | SMILES | C(Cl)/C=C/C#CC(C)(C)C | | CAS DataBase Reference | 287471-30-1(CAS DataBase Reference) |
| Provider | Language |
|
ALFA
| English |
| | 1-CHLORO-6,6-DIMETHYL-2-HEPTEN-4-YNE Usage And Synthesis |
| Description | 1-Chloro-6,6-dimethyl-2-hepten-4-yne is the allylamine antifungal agents terbinafine intermediate.1-Chloro-6,6-dimethyl-2-hepten-4-yne choose to inhibit COX-fungal squalene, so that reduction of membrane ergosterol, thus inhibiting the growth of fungi play an inhibitory role; right ergosterol synthesis of precursors and no inhibitory effect on other stages. | | Uses | 1-Chloro-6,6-dimethyl-2-heptene-4-yne is a hydrocarbon derivative and can be used as a pharmaceutical intermediate. | | Synthesis | 1-Chloro-6,6-dimethyl-2-hepten-4-yne mainly due to keratin aggregation produce squalene, was squalene cyclooxygenase inhibition, squalene in the cells of a large number gathered in the form of lipid droplets penetrate fungal cell membrane, destruction of the membrane lipid composition, resulting in fungal death. A typical procedure for preparing of 1-chloro-6,6-dimethyl-2-hepten-4-yne using boron trichloride is as follows: Compound 1 (53 g, 0.38 mol) in 2800 mL of n-hexane was cooled to 10??15??. Boron trichloride (1 M in hexane, 480 mL, 0.48 mol) was added to the mixture at 15??20?? over a 10 min period. After 10 min of stirring at 20??, the mixture was quenched with 1000 mL of water and stirred for 10 min. The separated organic phase was washed with 20% NaCl, dried (MgSO4), and evaporated to afford the title compound 2 (57.1 g, 95%) in 9:1 E:Z ratio.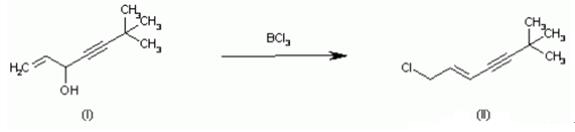 |
| | 1-CHLORO-6,6-DIMETHYL-2-HEPTEN-4-YNE Preparation Products And Raw materials |
|